| |

| |
| Names | |
|---|---|
| Other names
bisnickel bis(dithiobenzil)nickel(II) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.044.853 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H20NiS4 |
| Molar mass | 543.40 g·mol |
| Appearance | black-green solid |
| Density | 1.466 g/cm |
| Melting point | 260 °C (500 °F; 533 K) |
| Structure | |
| Crystal structure | monoclinic |
| Space group | P21/n |
| Lattice constant | a = 0.5836 nm, b = 1.097 nm, c = 1.836 nmα = 90°, β = 91.4°, γ = 90° |
| Hazards | |
| GHS labelling: | |
| Hazard statements | H317, H334, H350, H372 |
| Precautionary statements | P260, P270, P272, P280 |
| Safety data sheet (SDS) | Bis(dithiobenzil)nickel(II). TCI |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
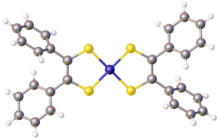
Nickel bis(stilbenedithiolate) or bis(dithiobenzil)nickel is a coordination complex with the formula Ni(S2C2Ph2)2 (where Ph = phenyl). It exists as a black solid that gives green solutions in toluene due to a strong absorption at 855 nm. The complex is a prototype of a large family of bis(dithiolene) complexes or the formula Ni(S2C2R2)2 (R = H, alkyl, aryl). These complexes have attracted much attention as dyes. They are of academic interest because the dithiolenes are noninnocent ligands. The lengths of the C-S and C-C bonds in the backbone, respectively 1.71 and 1.39 Å, are intermediate between double and single bonds.
The complex was prepared originally by treating nickel sulfide with diphenylacetylene. High yielding syntheses involve treating nickel salts with sulfided benzoin. The complex reacts with ligands to form monodithiolene complexes of the type Ni(S2C2Ph2)L2.
References
- Sartain, D.; Truter, Mary R. (1967). "The crystal structure of bis(dithiobenzil)nickel". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1264. doi:10.1039/J19670001264.
- Karlin, K. D.; Stiefel, E. I., Eds. “Progress in Inorganic Chemistry, Dithiolene Chemistry: Synthesis, Properties, and Applications” Wiley-Interscience: New York, 2003. ISBN 0-471-37829-1
- Miao, Qingqing; Gao, Junxiong; Wang, Zeqing; Yu, Hang; Luo, Yi; Ma, Tingli (2011). "Syntheses and Characterization of Several Nickel Bis(dithiolene) Complexes with Strong and Broad Near-IR Absorption". Inorganica Chimica Acta. 376: 619–627. doi:10.1016/j.ica.2011.07.046.
- Schrauzer, G. N.; Mayweg, V. (1962). "Reaction of Diphenylacetylene with Nickel Sulfides". Journal of the American Chemical Society. 84 (16): 3221. doi:10.1021/ja00875a061.
- Obanda, Antony; Martinez, Kristina; Schmehl, Russell H.; Mague, Joel T.; Rubtsov, Igor V.; MacMillan, Samantha N.; Lancaster, Kyle M.; Sproules, Stephen; Donahue, James P. (2017). "Expanding the Scope of Ligand Substitution from [M(S2C2Ph2] (M = Ni, Pd, Pt) to Afford New Heteroleptic Dithiolene Complexes" (PDF). Inorganic Chemistry. 56 (17): 10257–10267. doi:10.1021/acs.inorgchem.7b00971. PMID 28820242.
