 | |
| Names | |
|---|---|
| IUPAC name nickel(2+) diformate | |
| Systematic IUPAC name nickel formate | |
| Other names nickel diformate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.020.093 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H2NiO4 |
| Molar mass | 148.73 |
| Appearance | Green Solid |
| Odor | odourless |
| Density | 2.154 g/cm |
| Melting point | 130–140°C |
| Boiling point | 180–200°C (decomposition) |
| Solubility in water | Slightly soluble in cold water |
| Solubility | insoluble in organic solvents soluble in acids |
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H317, H334, H341, H350i, H360D, H372, H410 |
| Precautionary statements | P260, P285, P302+P352, P321, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
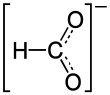
Nickel formate is the nickel salt of formic acid with the chemical formula Ni(HCOO)2.
Synthesis and structure
Nickel formate can be obtained by reacting nickel(II) acetate or nickel(II) hydroxide with formic acid.
- Ni(OH)2 + 2HCOOH → Ni(HCOO)2 + 2 H2O
Nickel formate can also be synthesized by the reaction of sodium formate with nickel (II) sulphate.
Characteristics
As a dihydrate, nickel formate is a green, odorless, non-flammable solid that is sparingly soluble in water. The compound has a monoclinic crystal structure. The anhydride forms on careful heating at 130–140 °C. When heated in a vacuum to 300 °C, pure nickel is formed:
- Ni(HCO2)2(H2O)2 → Ni + 2 CO2 + 2 H2O + H2
Such fine powders are useful as hydrogenation catalysts.
Use
Nickel formate is used in the production of nickel and other nickel compounds such as nickel catalysts.
References
- "Nickel formate".
- ^ Record of Nickeldiformat in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2016-07-23.
- ^ NLM Hazardous Substances Data Bank entry for
- ^ Milne, G. W. A. (2005). Gardner's Commercially Important Chemicals Synonyms, Trade Names, and Properties. John Wiley & Sons. p. 738. ISBN 0-471-73661-9.
- ^ Kotz, John; Treichel, Paul; Townsend, John (2009). Chemistry and Chemical Reactivity, Enhanced Edition. Cengage Learning. p. 335. ISBN 978-0-495-39029-9.
- Falbe, Jürgen; Regitz, Manfred (2014). RÖMPP Lexikon Chemie, 10. Auflage, 1996-1999 Band 4: M - Pk. Georg Thieme Verlag. p. 2238. ISBN 978-3-13-200031-5.
- Werner Reutemann and Heinz Kieczka "Formic Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_013