
In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is RRC=N(−O)(−R), where R is not a hydrogen. Their primary application is intermediates in chemical synthesis. A nitrone is a 1,3-dipole used in cycloadditions, and a carbonyl mimic.
Structure
Nitrones, as a tetrasubstituted double bond, admit cis–trans isomerism.
Generation of nitrones
Typical nitrone sources are hydroxylamine oxidation or condensation with carbonyl compounds. Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate. The most general reagent used for the oxidation of hydroxylamines is aqueous mercuric oxide:
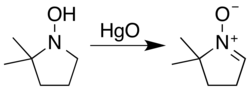
However, a hydroxylamine with two α hydrogens may unsaturate on either side. Carbonyl condensation avoids this ambiguity...

...but is inhibited if both ketone substituents are bulky.
In principle, N-alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack.
Reactions
Some nitrones oligomerize:
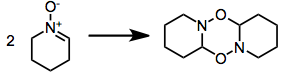
Syntheses with nitrone precursors obviate the issue with increased temperature, to exaggerate entropic factors; or with a nitrone excess.
Carbonyl mimic
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction. The α carbon is an electrophile and the β carbon a nucleophile; that is, nitrones polarize like carbonyls and nitriles but unlike nitro compounds and vinyl sulfur derivatives.
Nitrones hydrolyze extremely easily to the corresponding carbonyl and N-hydroxylamine.
1,3-dipolar cycloadditions
Main article: Nitrone-olefin 3+2 cycloadditionAs 1,3‑dipoles, nitrones perform cycloadditions. For example, a dipolarophilic alkene combines to form isoxazolidine:

Other ring-closing reactions are known, including formal and cycloadditions.
Isomerization
Deoxygenating reagents, light, or heat all catalyze rearrangement to the amide. Acids catalyze rearrangement to the oxime ether.
Reduction
Hydrides add to give hydroxylamines. Reducing Lewis acids (e.g. metals, SO2) deoxygenate to the imine instead.
See also
References
- ^ Hamer, Jan; Macaluso, Anthony (1964-08-01). "Nitrones". Chemical Reviews. 64 (4): 473–495. doi:10.1021/cr60230a006. ISSN 0009-2665.
- ^ Delpierre, G. R.; Lamchen, M. (1965). "Nitrones". Quarterly Reviews, Chemical Society. 19 (4): 329. doi:10.1039/qr9651900329. ISSN 0009-2681.
- Thiesing, Jan; Mayer, Hans (1957). "Cyclische Nitrone, II. Über die Polymeren des 2.3.4.5-Tetrahydro-pyridin-N-oxyds und verwandte Verbindungen". Justus Liebigs Ann. Chem. 609: 46-57. doi:10.1002/jlac.19576090105.
- Exner, O. (1951). "A New Synthesis of N-methylketoximes". ChemPlusChem. 16: 258-267. doi:10.1135/cccc19510258.
- Thiesing, Jan; Mayer, Hans (1956). "Cyclische Nitrone I: Dimeres 2.3.4.5-Tetrahydro-pyridin-N-oxyd". Chem. Ber. 89 (9): 2159-2167. doi:10.1002/cber.19560890919.
- ^ Yang, Jiong (2012). "Recent Developments in Nitrone Chemistry". Synlett. 23: 2293-97. doi:10.1055/s-0032-1317096.
- Murahashi, Shun-Ichi; Imada, Yasushi (15 March 2019). "Synthesis and Transformations of Nitrones for Organic Synthesis". Chemical Reviews. 119 (7): 4684–4716. doi:10.1021/acs.chemrev.8b00476. PMID 30875202. S2CID 80623450.