
| |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Bicycloheptane | |
| Other names norcamphane, norbornylane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1900379 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.452 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H12 |
| Molar mass | 96.17 g mol |
| Appearance | white volatile solid |
| Melting point | 85 to 88 °C (185 to 190 °F; 358 to 361 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
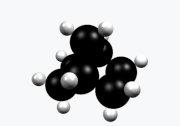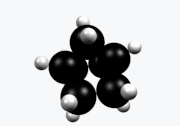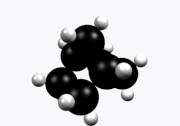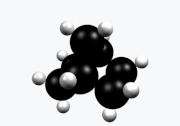
Norbornane (also known as bicycloheptane) is an organic compound and a saturated hydrocarbon with chemical formula C7H12. It is a crystalline compound with a melting point of 88 °C. The carbon skeleton is derived from cyclohexane ring with a methylene bridge in the 1,4- position, and is a bridged bicyclic compound. The compound is a prototype of a class of strained bicyclic hydrocarbons.
The compound was originally synthesized by reduction of norcamphor.
The name norbornane is derived from bornane, which is 1,7,7-trimethylnorbornane, being a derivative of camphor (bornanone). The prefix nor refers to the stripping of the methyl groups from the parent molecule bornane.
See also
- 2-Norbornyl cation
- Norbornene
- Norbornadiene
- Bornane
- endo-Norborneol
- exo-Norborneol
- Norcamphor, the ketone derivative of norbornane
References
- Komppa, Gust.; Beckmann, Siegfried (1934). "Der Grundkörper der Camphergruppe, das Bicyclo--heptan, und die stereoisomeren Norborneole". Naturwissenschaften. 22: 171. doi:10.1007/BF01496254.