 | |
| Clinical data | |
|---|---|
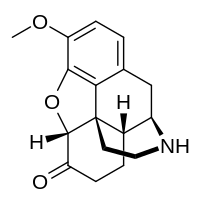
| Other names | (5α)-3-Methoxy-4,5-epoxymorphinan-6-one |
| Dependence liability | High |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H19NO3 |
| Molar mass | 285.343 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Norhydrocodone is the major metabolite of the opioid analgesic hydrocodone. It is formed from hydrocodone in the liver via N-demethylation predominantly by CYP3A4. Unlike hydromorphone, a minor metabolite of hydrocodone, norhydrocodone is described as inactive. However, norhydrocodone is actually an agonist of the μ-opioid receptor with similar potency to hydrocodone, but has been found to produce only minimal analgesia when administered peripherally to animals. This is likely due to poor blood-brain-barrier and thus central nervous system penetration.
See also
References
- ^ Zhou S (6 April 2016). Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. pp. 164–. ISBN 978-1-4665-9788-4.
- Dasgupta A, Langman LJ (23 April 2012). Pharmacogenomics of Alcohol and Drugs of Abuse. CRC Press. pp. 175–. ISBN 978-1-4398-5611-6.
- ^ Navani DM, Yoburn BC (November 2013). "In vivo activity of norhydrocodone: an active metabolite of hydrocodone". The Journal of Pharmacology and Experimental Therapeutics. 347 (2): 497–505. doi:10.1124/jpet.113.207548. PMID 23995596. S2CID 31072872.
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |