 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H18FNOS |
| Molar mass | 303.40 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
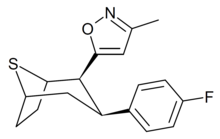
O-4210 is a drug developed by Organix Inc which acts as a selective dopamine reuptake inhibitor, with good selectivity over the serotonin transporter but its activity at the noradrenaline transporter is not known. It is a thiatropane derivative, related in chemical structure to phenyltropane derivatives such as RTI-126 and RTI-171, but with the amine nitrogen replaced by sulfur, demonstrating that this nitrogen only plays a minor contribution to receptor binding, in a similar manner to the related oxatropane tropoxane.
See also
References
- Pham-Huu DP, Deschamps JR, Liu S, Madras BK, Meltzer PC (January 2007). "Synthesis of 8-thiabicyclo[3.2.1]octanes and their binding affinity for the dopamine and serotonin transporters". Bioorganic & Medicinal Chemistry. 15 (2): 1067–82. doi:10.1016/j.bmc.2006.10.016. PMC 1829488. PMID 17070057.
- Purushotham M, Sheri A, Pham-Huu DP, Madras BK, Janowsky A, Meltzer PC (January 2011). "The synthesis and biological evaluation of 2-(3-methyl or 3-phenylisoxazol-5-yl)-3-aryl-8-thiabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 21 (1): 48–51. doi:10.1016/j.bmcl.2010.11.076. PMC 3015105. PMID 21146984.
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |