| |||
| Names | |||
|---|---|---|---|
| IUPAC name 2,2,4,4,6,6,8,8-Octachloro-1,3,5,7,2λ,4λ,6λ,8λ-tetrazatetraphosphocine | |||
| Other names
Phosphonitrilic chloride tetramer Octachlorocyclotetraphosphazene Tetraphosphonitrilic chloride 2,2,4,4,6,6,8,8-octachloro-2,2,4,4,6,6,8,8-octahydro-1,3,5,7,2,4,6,8-tetraazatetraphosphocine 2,2,4,4,6,6,8,8-octachloro-1,3,5,7-tetraza-2λ,4λ,6λ,8λ-tetraphosphacycloocta-1,3,5,7-tetraene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | N4Cl8P4 | ||
| Molar mass | 463.55 g/mol | ||
| Appearance | Colorless solid | ||
| Density | 2.27 g/mL at -173 °C | ||
| Melting point | 123 to 124 °C (253 to 255 °F; 396 to 397 K) | ||
| Boiling point | 188 °C at 15 Torr | ||
| Solubility in water | Decomposes | ||
| Solubility in hexane | 7.0 g/100g (20 °C) | ||
| Solubility in toluene | 1.8 g/100g (20 °C) | ||
| Solubility in CCl4 | 1.6 g/100g (20 °C) | ||
| Refractive index (nD) | 1.675 (589 nm) | ||
| Structure | |||
| Crystal structure | Tetragonal | ||
| Space group | 86 | ||
| Point group | D4h | ||
| Dipole moment | 0.39 D | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
-1084.9 kJ·mol | ||
| Enthalpy of vaporization (ΔfHvap) | 64.9 kJ·mol (325 °C) | ||
| Enthalpy of sublimation (ΔfHsublim) | 95.9–97.5 kJ·mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Mild irritant | ||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Danger | ||
| Hazard statements | H314 | ||
| Precautionary statements | P260, P280, P304+P340, P305+P351+P338, P363 | ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Related compounds | Hexachlorophosphazene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
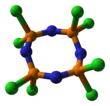
Octachlorotetraphosphazene is an inorganic compound with the formula (NPCl2)4. The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen centers, and can be viewed as a tetramer of the hypothetical compound N≡PCl2.
The compound has not been studied as much as the related species hexachlorotriphosphazene, in the samples of which octachlorotetraphosphazene is usually found as an unwanted contamintant.
Structure and bonding
Octachlorotetraphosphazene has a P4N4 core with six equivalent P–N bonds.
Synthesis
- NH4Cl + PCl5 → 1/n (NPCl2)n + HCl
Reactions
Substitution at P
Some spiro-, ansa-, and spiro-ansa-cyclic derivatives have been prepared via nucleophilic substitution of octachlorotetraphosphazene with alkoxides.
References
- Allcock, H. R. (1972). Phosphorus-nitrogen compounds ; cyclic, linear, and high polymeric systems. New York: Academic Press. ISBN 978-0-323-14751-4. OCLC 838102247.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Ture, Sedat (2016-01-02). "Synthesis and characterization of spiro-, ansa-, and spiro-ansa-cyclic derivatives of cyclotetraphosphazene with the reactions of pentane-1,5-diol". Phosphorus, Sulfur, and Silicon and the Related Elements. 191 (1): 129–139. doi:10.1080/10426507.2015.1054483.