| |
| Names | |
|---|---|
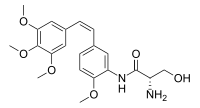
| IUPAC name N-{2-Methoxy-5-phenyl}-L-serinamide | |
| Systematic IUPAC name (2S)-2-Amino-3-hydroxy-N-{2-methoxy-5-phenyl}propanamide | |
| Other names AVE-8062; AVE-8062A; AC7700; CS-39-L-Ser.HCl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H26N2O6 |
| Molar mass | 402.447 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ombrabulin was an experimental drug candidate discovered by Ajinomoto and further developed by Sanofi-Aventis. Ombrabulin is a combretastatin A-4 derivative that exerts its antitumor effect by disrupting the formation of blood vessels needed for tumor growth.
It was granted orphan drug status by the European Medicines Agency in April 2011.
In January 2013, Sanofi said it discontinued development of ombrabulin after disappointing results from phase III clinical trials.
References
- "Ombrabulin (AVE8062)". Sanofi-Aventis Oncology.
- Hori, K; Saito, S; Nihei, Y; Suzuki, M; Sato, Y (1999). "Antitumor effects due to irreversible stoppage of tumor tissue blood flow: evaluation of a novel combretastatin A-4 derivative, AC7700". Japanese Journal of Cancer Research. 90 (9): 1026–38. doi:10.1111/j.1349-7006.1999.tb00851.x. PMC 5926172. PMID 10551334.
- Hori, K; Saito, S; Kubota, K (2002). "A novel combretastatin A-4 derivative, AC7700, strongly stanches tumour blood flow and inhibits growth of tumours developing in various tissues and organs". British Journal of Cancer. 86 (10): 1604–14. doi:10.1038/sj.bjc.6600296. PMC 2746587. PMID 12085211.
- "Orphan Designation EU/3/11/853". European Medicines Agency. 15 April 2011.
- "Sanofi has 65 new compounds in development, says R&D chief".