| |

| |
| Names | |
|---|---|
| Other names Octabutyltetrathiocyanatostannoxane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C36H72N4O2S4Sn4 |
| Molar mass | 1196.08 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
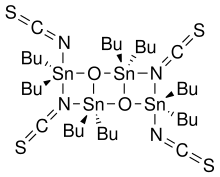
Otera's catalyst, named after Japanese chemist Junzo Otera, is an organostannane compound which has been used as a transesterification catalyst. This isothioscyanate compound is a member of a family of organostannanes reported by Wada and coworkers, and elaborated upon by Otera and coworkers.
Preparation
This class of compounds may be prepared generally by the reaction of an organotin halide and oxide:
- 2 R2SnO + 2 R2SnX2 → (XR2SnOSnR2X)2
In particular, the thiocyanate compound was prepared by the reaction of dibutyltin oxide with dibutyltin diisothiocyanate. Otherwise, this compound is not commercially available.
Applications
This thiocyanate compound can be used as a transesterification catalyst. Although it is not well known, it has been used in a number of total syntheses.
In this application, the reaction occurs via the displacement of the bridging isothiocyanate ligands with the incoming alcohol to form an alcohol-bridged active catalyst. Tin acts as the Lewis acid, and gives the transesterified product.
References
- ^ Wada, M.; Nishino, M.; Okawara, R. (1965). "Preparation and properties of dialkyltin isothiocyanate derivatives". J. Organomet. Chem. 3: 70–75. doi:10.1016/S0022-328X(00)82737-0.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Otera, J; et al. (1991). "Novel template effects of distannoxane catalysts in highly efficient transesterification and esterification". J. Org. Chem. 56 (18): 5307–5311. doi:10.1021/jo00018a019.
- ^ Otera, Junzo. (1993). "Transesterification". Chem. Rev. 93 (4): 1449–1470. doi:10.1021/cr00020a004.
- Trost, BM; et al. (2005). "Synthesis of Amphidinolide P". J. Am. Chem. Soc. 127 (50): 17921–17937. doi:10.1021/ja055967n. PMC 2533515. PMID 16351124.
- Trost, BM; Stiles, DT (2007). "Total Synthesis of Spirotryprostatin B via Diastereoselective Prenylation". Org. Lett. 9 (15): 2763–6. doi:10.1021/ol070971k. PMID 17592853.