| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
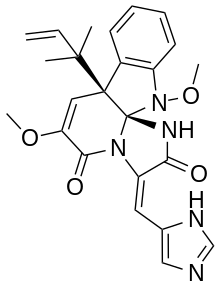
| Chemical formula | C24H25N5O4 |
| Molar mass | 447.495 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Oxaline is a fungal isolate with anticancer activity in vitro. It is an O-methylated derivative of meleagrin.
References
- Koizumi, Y; Arai, M; Tomoda, H; Omura, S (2004). "Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1693 (1): 47–55. doi:10.1016/j.bbamcr.2004.04.013. PMID 15276324.
This article about an alkaloid is a stub. You can help Misplaced Pages by expanding it. |