| |

| |
| Names | |
|---|---|
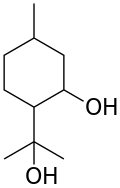
| Preferred IUPAC name 2-(2-Hydroxypropan-2-yl)-5-methylcyclohexan-1-ol | |
| Other names
2-(1-Hydroxy-1-methylethyl)-5-methylcyclohexanol para-Menthane-3,8-diol 2-Hydroxy-α,α,4-trimethylcyclohexanemethanol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 2552262 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.050.849 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H20O2 |
| Molar mass | 172.268 g·mol |
| Density | 1.009 g/cm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
p-Menthane-3,8-diol, also known as para-menthane-3,8-diol, PMD, or menthoglycol, is an organic compound classified as a diol and a terpenoid. It is colorless. Its name reflects the hydrocarbon backbone, which is that of p-menthane. A total of eight stereoisomers are possible, based on the three stereocenters of the ring. Depending on the source, one or more may predominate.
PMD is the active ingredient in some insect repellents. Its odor and chemical structure are similar to menthol, and it has a cooling feel. It is found in small quantities in the essential oil from the leaves of Corymbia citriodora, formerly known as Eucalyptus citriodora. This tree is native to Australia, but is now cultivated in many warm places around the world. C. citriodora oil, when refined to increase its PMD content for use in insect repellents, is known in the United States as oil of lemon eucalyptus (OLE). C. citriodora oil contains only 1–2% PMD, while refined OLE contains approximately up to 70% PMD. Some commercial PMD products are not made from C. citriodora oil, but rather from synthetic citronellal.
Effectiveness
A 2006 study showed that PMD used as a repellent is as effective as DEET when used in like quantities.
Contraindications
PMD should not be used on children under 3 years of age.
Interactions
Few if any studies have evaluated possible interactions when using PMD with sunscreens.
Chemistry
There are eight possible stereoisomers. The exact composition is rarely specified and is commonly assumed to be a complex mixture. PMD can be synthesized by a Prins reaction of citronellal.
Refined OLE contains approximately up to 70% PMD, a mixture of the cis and trans isomers of p-menthane-3,8-diol.
History
Its repellent effect was discovered in the 1960s by the industry.
OLE has been notified under the European Biocidal Products Directive (BPD) 98/8/EC (now BPR Regulation (EU) No. 528/212) under its generic name "PMD rich botanic oil" and is currently proceeding through the registration process with the Health and Safety Executive in the UK. It is also registered with Canada's Pest Management Regulatory Agency under the generic name "PMD and related oil of lemon eucalyptus compounds".
See also
- DEET
- Ethyl butylacetylaminopropionate (IR3535)
- Icaridin
- Permethrin, a pyrethroid insecticide that can be applied to clothing to help prevent bites
References
- John C. Leffingwell. "Cool without menthol & cooler than menthol and cooling compounds as insect repellents". Leffingwell & Associates.
- "Home". citrepel.co.uk.
- ^ Drapeau, Jeremy (2011). "Green synthesis of para-Menthane-3,8-diol from Eucaplytus citriodora: Application for repellent products". Comptes Rendus Chimie. 14 (7–8): 629–635. doi:10.1016/j.crci.2011.02.008.

- Carroll, Scott P.; Loye, Jenella (2006). "PMD, a Registered Botanical Mosquito Repellent with Deet-Like Efficacy". Journal of the American Mosquito Control Association. 22 (3): 507–13. doi:10.2987/8756-971X(2006)22[507:PARBMR]2.0.CO;2. PMID 17067054. S2CID 225645.
- "Prevent Mosquito Bites". Centers for Disease Control and Prevention. Retrieved 24 September 2023.
- "Insect Repellents". National Pesticide Information Center. February 2018. Archived from the original on 13 September 2023. Retrieved 26 September 2023.
Few or no studies address using IR3535 or oil of lemon eucalyptus with sunscreens.
- PubChem Compound Search