| PA clan of proteases | |
|---|---|
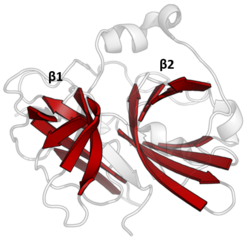 The double β-barrels that characterise the PA clan are highlighted in red. (TEV protease, PDB: 1lvm) The double β-barrels that characterise the PA clan are highlighted in red. (TEV protease, PDB: 1lvm) | |
| Identifiers | |
| Symbol | N/A |
| Pfam clan | CL0124 |
| ECOD | 1.1.5 |
| InterPro | IPR009003 |
| SCOP2 | 50494 / SCOPe / SUPFAM |
| Membranome | 319 |
The PA clan (Proteases of mixed nucleophile, superfamily A) is the largest group of proteases with common ancestry as identified by structural homology. Members have a chymotrypsin-like fold and similar proteolysis mechanisms but can have identity of <10%. The clan contains both cysteine and serine proteases (different nucleophiles). PA clan proteases can be found in plants, animals, fungi, eubacteria, archaea and viruses.
The common use of the catalytic triad for hydrolysis by multiple clans of proteases, including the PA clan, represents an example of convergent evolution. The differences in the catalytic triad within the PA clan is also an example of divergent evolution of active sites in enzymes.
History
In the 1960s, the sequence similarity of several proteases indicated that they were evolutionarily related. These were grouped into the chymotrypsin-like serine proteases (now called the S1 family). As the structures of these, and other proteases were solved by X-ray crystallography in the 1970s and 80s, it was noticed that several viral proteases such as Tobacco Etch Virus protease showed structural homology despite no discernible sequence similarity and even a different nucleophile. Based on structural homology, a superfamily was defined and later named the PA clan (by the MEROPS classification system). As more structures are solved, more protease families have been added to the PA clan superfamily.
Etymology
The P refers to Proteases of mixed nucleophile. The A indicates that it was the first such clan to be identified (there also exist the PB, PC, PD and PE clans).
Structure
 Structural homology in the PA superfamily. The double beta-barrel that characterises the superfamily is highlighted in red. Shown are representative structures from several families within the PA superfamily. Note that some proteins show partially modified structural. Chymotrypsin (PDB: 1gg6), thrombin (PDB: 1mkx), tobacco etch virus protease (PDB: 1lvm), calicivirin (PDB: 1wqs), west nile virus protease (PDB: 1fp7), exfoliatin toxin (PDB: 1exf), HtrA protease (PDB: 1l1j), snake venom plasminogen activator (PDB: 1bqy), chloroplast protease (PDB: 4fln) and equine arteritis virus protease (PDB: 1mbm).
Structural homology in the PA superfamily. The double beta-barrel that characterises the superfamily is highlighted in red. Shown are representative structures from several families within the PA superfamily. Note that some proteins show partially modified structural. Chymotrypsin (PDB: 1gg6), thrombin (PDB: 1mkx), tobacco etch virus protease (PDB: 1lvm), calicivirin (PDB: 1wqs), west nile virus protease (PDB: 1fp7), exfoliatin toxin (PDB: 1exf), HtrA protease (PDB: 1l1j), snake venom plasminogen activator (PDB: 1bqy), chloroplast protease (PDB: 4fln) and equine arteritis virus protease (PDB: 1mbm).
 Above, sequence conservation of 250 members of the PA protease clan (superfamily). Below, sequence conservation of 70 members of the C04 protease family. Arrows indicate catalytic triad residues. Aligned on the basis of structure by DALI
Above, sequence conservation of 250 members of the PA protease clan (superfamily). Below, sequence conservation of 70 members of the C04 protease family. Arrows indicate catalytic triad residues. Aligned on the basis of structure by DALI
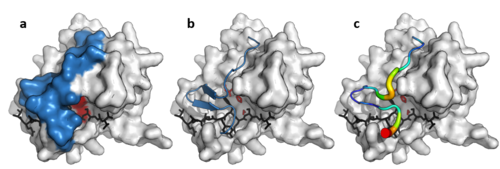
Despite retaining as little as 10% sequence identity, PA clan members isolated from viruses, prokaryotes and eukaryotes show structural homology and can be aligned by structural similarity (e.g. with DALI).
Double β-barrel
PA clan proteases all share a core motif of two β-barrels with covalent catalysis performed by an acid-histidine-nucleophile catalytic triad motif. The barrels are arranged perpendicularly beside each other with hydrophobic residues holding them together as the core scaffold for the enzyme. The triad residues are split between the two barrels so that catalysis takes place at their interface.
Viral protease loop
In addition to the double β-barrel core, some viral proteases (such as TEV protease) have a long, flexible C-terminal loop that forms a lid that completely covers the substrate and create a binding tunnel. This tunnel contains a set of tight binding pockets such that each side chain of the substrate peptide (P6 to P1’) is bound in a complementary site (S6 to S1’) and specificity is endowed by the large contact area between enzyme and substrate. Conversely, cellular proteases that lack this loop, such as trypsin have broader specificity.
Evolution and function
See also: Catalytic triad § Comparison of serine and cysteine hydrolase mechanismsCatalytic activity
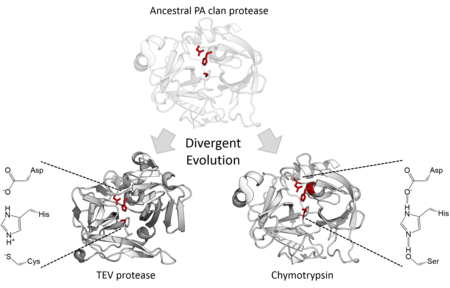
Structural homology indicates that the PA clan members are descended from a common ancestor of the same fold. Although PA clan proteases use a catalytic triad perform 2-step nucleophilic catalysis, some families use serine as the nucleophile whereas others use cysteine. The superfamily is therefore an extreme example of divergent enzyme evolution since during evolutionary history, the core catalytic residue of the enzyme has switched in different families. In addition to their structural similarity, directed evolution has been shown to be able to convert a cysteine protease into an active serine protease. All cellular PA clan proteases are serine proteases, however there are both serine and cysteine protease families of viral proteases. The majority are endopeptidases, with the exception being the S46 family of exopeptidases.
Biological role and substrate specificity
In addition to divergence in their core catalytic machinery, the PA clan proteases also show wide divergent evolution in function. Members of the PA clan can be found in eukaryotes, prokaryotes and viruses and encompass a wide range of functions. In mammals, some are involved in blood clotting (e.g. thrombin) and so have high substrate specificity as well as digestion (e.g. trypsin) with broad substrate specificity. Several snake venoms are also PA clan proteases, such as pit viper haemotoxin and interfere with the victim's blood clotting cascade. Additionally, bacteria such as Staphylococcus aureus secrete exfoliative toxin which digest and damage the host's tissues. Many viruses express their genome as a single, massive polyprotein and use a PA clan protease to cleave this into functional units (e.g. polio, norovirus, and TEV proteases).
There are also several pseudoenzymes in the superfamily, where the catalytic triad residues have been mutated and so function as binding proteins. For example, the heparin-binding protein Azurocidin has a glycine in place of the nucleophile and a serine in place of the histidine.
Families
Within the PA clan (P=proteases of mixed nucleophiles), families are designated by their catalytic nucleophile (C=cysteine proteases, S=serine proteases). Despite the lack of sequence homology for the PA clan as a whole, individual families within it can be identified by sequence similarity.
See also
- Protease
- Catalytic triad
- Homology (biology)
- MEROPS
- Protein family
- Protein superfamily
- Protein structure
- Structural alignment
References
- ^ Rawlings ND, Barrett AJ, Bateman A (January 2012). "MEROPS: the database of proteolytic enzymes, their substrates and inhibitors". Nucleic Acids Research. 40 (Database issue): D343-50. doi:10.1093/nar/gkr987. PMC 3245014. PMID 22086950.
- ^ Bazan JF, Fletterick RJ (November 1988). "Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications". Proceedings of the National Academy of Sciences of the United States of America. 85 (21): 7872–6. Bibcode:1988PNAS...85.7872B. doi:10.1073/pnas.85.21.7872. PMC 282299. PMID 3186696.
- ^ Laskar A, Rodger EJ, Chatterjee A, Mandal C (May 2012). "Modeling and structural analysis of PA clan serine proteases". BMC Research Notes. 5: 256. doi:10.1186/1756-0500-5-256. PMC 3434108. PMID 22624962.
- Barbosa JA, Saldanha JW, Garratt RC (July 1996). "Novel features of serine protease active sites and specificity pockets: sequence analysis and modelling studies of glutamate-specific endopeptidases and epidermolytic toxins". Protein Engineering. 9 (7): 591–601. doi:10.1093/protein/9.7.591. PMID 8844831.
- "MEROPS - Archaeal S01 proteases".
- Ruiz-Perez F, Nataro JP (March 2014). "Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence". Cellular and Molecular Life Sciences. 71 (5): 745–70. doi:10.1007/s00018-013-1355-8. PMC 3871983. PMID 23689588.
- ^ Buller AR, Townsend CA (February 2013). "Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad". Proceedings of the National Academy of Sciences of the United States of America. 110 (8): E653-61. Bibcode:2013PNAS..110E.653B. doi:10.1073/pnas.1221050110. PMC 3581919. PMID 23382230.
- de Haën C, Neurath H, Teller DC (February 1975). "The phylogeny of trypsin-related serine proteases and their zymogens. New methods for the investigation of distant evolutionary relationships". Journal of Molecular Biology. 92 (2): 225–59. doi:10.1016/0022-2836(75)90225-9. PMID 1142424.
- Lesk AM, Fordham WD (May 1996). "Conservation and variability in the structures of serine proteinases of the chymotrypsin family". Journal of Molecular Biology. 258 (3): 501–37. doi:10.1006/jmbi.1996.0264. PMID 8642605.
- Gorbalenya AE, Blinov VM, Donchenko AP (January 1986). "Poliovirus-encoded proteinase 3C: a possible evolutionary link between cellular serine and cysteine proteinase families". FEBS Letters. 194 (2): 253–7. Bibcode:1986FEBSL.194..253G. doi:10.1016/0014-5793(86)80095-3. PMID 3000829. S2CID 23268152.
- ^ Phan J, Zdanov A, Evdokimov AG, Tropea JE, Peters HK, Kapust RB, Li M, Wlodawer A, Waugh DS (December 2002). "Structural basis for the substrate specificity of tobacco etch virus protease". The Journal of Biological Chemistry. 277 (52): 50564–72. doi:10.1074/jbc.M207224200. PMID 12377789.
- Allaire M, Chernaia MM, Malcolm BA, James MN (May 1994). "Picornaviral 3C cysteine proteinases have a fold similar to chymotrypsin-like serine proteinases". Nature. 369 (6475): 72–6. Bibcode:1994Natur.369...72A. doi:10.1038/369072a0. PMID 8164744. S2CID 4312593.
- Snijder EJ, Wassenaar AL, van Dinten LC, Spaan WJ, Gorbalenya AE (March 1996). "The arterivirus nsp4 protease is the prototype of a novel group of chymotrypsin-like enzymes, the 3C-like serine proteases". The Journal of Biological Chemistry. 271 (9): 4864–71. doi:10.1074/jbc.271.9.4864. PMID 8617757.
- Dougherty WG, Parks TD, Cary SM, Bazan JF, Fletterick RJ (September 1989). "Characterization of the catalytic residues of the tobacco etch virus 49-kDa proteinase". Virology. 172 (1): 302–10. doi:10.1016/0042-6822(89)90132-3. PMID 2475971.
- Laskar A, Rodger EJ, Chatterjee A, Mandal C (May 2012). "Modeling and structural analysis of PA clan serine proteases". BMC Research Notes. 5 (1): 256. doi:10.1186/1756-0500-5-256. PMC 3434108. PMID 22624962.
- Shafee T, Gatti-Lafranconi P, Minter R, Hollfelder F (September 2015). "Handicap-Recover Evolution Leads to a Chemically Versatile, Nucleophile-Permissive Protease". ChemBioChem. 16 (13): 1866–1869. doi:10.1002/cbic.201500295. PMC 4576821. PMID 26097079.
- Suzuki Y, Sakamoto Y, Tanaka N, Okada H, Morikawa Y, Ogasawara W (March 2014). "Identification of the catalytic triad of family S46 exopeptidases, closely related to clan PA endopeptidases". Scientific Reports. 4: 4292. Bibcode:2014NatSR...4E4292S. doi:10.1038/srep04292. PMC 3944710. PMID 24598890.
- Sakamoto Y, Suzuki Y, Iizuka I, Tateoka C, Roppongi S, Fujimoto M, Inaka K, Tanaka H, Masaki M, Ohta K, Okada H, Nonaka T, Morikawa Y, Nakamura KT, Ogasawara W, Tanaka N (May 2014). "S46 peptidases are the first exopeptidases to be members of clan PA". Scientific Reports. 4: 4977. Bibcode:2014NatSR...4E4977S. doi:10.1038/srep04977. PMC 4021333. PMID 24827749.
- Salvesen G (2013). Rawlings N (ed.). Handbook of proteolytic enzymes. Boston: Academic Press. ISBN 9780123822192.
- Polgár L (October 2005). "The catalytic triad of serine peptidases". Cellular and Molecular Life Sciences. 62 (19–20): 2161–72. doi:10.1007/s00018-005-5160-x. PMC 11139141. PMID 16003488. S2CID 3343824.
- Todd AE, Orengo CA, Thornton JM (October 2002). "Sequence and structural differences between enzyme and nonenzyme homologs". Structure. 10 (10): 1435–51. doi:10.1016/s0969-2126(02)00861-4. PMID 12377129.
- Iversen LF, Kastrup JS, Bjørn SE, Rasmussen PB, Wiberg FC, Flodgaard HJ, Larsen IK (April 1997). "Structure of HBP, a multifunctional protein with a serine proteinase fold". Nature Structural Biology. 4 (4): 265–8. doi:10.1038/nsb0497-265. PMID 9095193. S2CID 19949043.
External links
- MEROPS Archived 2017-05-10 at the Wayback Machine - Comprehensive protease database
- Superfamily Archived 2016-06-24 at the Wayback Machine - A database of protein folds
| Hydrolase: proteases (EC 3.4) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.4.11-19: Exopeptidase |
| ||||||||||||||
| 3.4.21-25: Endopeptidase | |||||||||||||||
| 3.4.99: Unknown | |||||||||||||||
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|