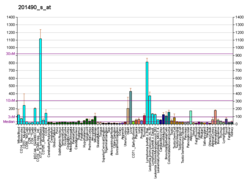
Peptidyl-prolyl cis-trans isomerase, mitochondrial (PPIF) is an enzyme that in humans is encoded by the PPIF gene. It has also been referred to as, but should not be confused with, cyclophilin D (CypD), which is encoded by the PPID gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to facilitate folding or repair of proteins. PPIF is a major component of the mitochondrial permeability transition pore (MPTP) and, thus, highly involved in mitochondrial metabolism and apoptosis, as well as in mitochondrial diseases and related conditions, including cardiac diseases, neurodegenerative diseases, and muscular dystrophy. In addition, PPIF participates in inflammation, as well as in ischemic reperfusion injury, AIDS, and cancer.
Structure
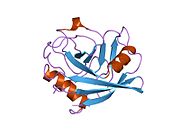
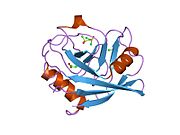
Like other cyclophilins, PPIF forms a β-barrel structure with a hydrophobic core. This β-barrel is composed of eight anti-parallel β-strands and capped by two α-helices at the top and bottom. In addition, the β-turns and loops in the strands contribute to the flexibility of the barrel. PPIF weighs 17.5 kDa and forms part of the MPTP in the inner mitochondrial membrane (IMM).
Function
The protein encoded by this gene is a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family. PPIases catalyze the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and accelerate the folding of proteins. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved. The PPIase family is further divided into three structurally distinct subfamilies: cyclophilin (CyP), FK506-binding protein (FKBP), and parvulin (Pvn). As a cyclophilin, PPI binds cyclosporin A (CsA) and can be found within the cell or secreted by the cell. In eukaryotes, cyclophilins localize ubiquitously to many cell and tissue types, though studies on PPIF focus primarily on heart, liver, and brain tissue. In addition to PPIase and protein chaperone activities, cyclophilins also function in mitochondrial metabolism, apoptosis, immunological response, inflammation, and cell growth and proliferation. PPIF is especially involved in mitochondrial apoptosis as a major component of the MPTP. Through its PPIase ability, the protein interacts with and induces a conformational change in adenine nucleotide translocase (ANT), the other MPTP component. This activation, along with high calcium ion levels, induces the opening the MPTP, resulting in mitochondrial swelling, increasing reactive oxygen species (ROS) levels, membrane depolarization, failing ATP production, caspase cascade activation, and ultimately, apoptosis.
Clinical significance
As a cyclophilin, PPIF binds the immunosuppressive drug CsA to form a CsA-cyclophilin complex, which then targets calcineurin to inhibit the signaling pathway for T-cell activation.
Due to its association with the MPTP, PPIF is also involved in neurodegenerative diseases, including glaucoma, diabetic retinopathy, Parkinson's disease, and Alzheimer's disease. For neurodegenerative diseases, treatment of reperfusion events with CsA, a PPID inhibitor, prevents cytochrome C release and significantly reduces cell death in neurons. As such, PPID proves to be an effective therapeutic target for patients suffering neurodegenerative diseases.
In addition, PPIF, as part of the MPTP, is involved in ischemia/reperfusion injury, traumatic brain injury (TBI), muscular dystrophy, and drug toxicity. Though PPIF was identified as a candidate for dilated cardiomyopathy (DCM) for one afflicted family, further study revealed no mutations in the gene to implicate it in the disease. Nonetheless, in cardiac myogenic cells, cyclophilins have been observed to be activated by heat shock and hypoxia-reoxygenation as well as complex with heat shock proteins. Thus, cyclophilins may function in cardioprotection during ischemia-reperfusion injury.
Currently, cyclophilin expression is highly correlated with cancer pathogenesis, but the specific mechanisms remain to be elucidated.
Interactions
PPIF has been shown to interact with:
References
- ^ GRCh38: Ensembl release 89: ENSG00000108179 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000021868 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Bergsma DJ, Eder C, Gross M, Kersten H, Sylvester D, Appelbaum E, Cusimano D, Livi GP, McLaughlin MM, Kasyan K (Dec 1991). "The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms". The Journal of Biological Chemistry. 266 (34): 23204–14. doi:10.1016/S0021-9258(18)54484-7. PMID 1744118.
- ^ "Entrez Gene: PPIF peptidylprolyl isomerase F (cyclophilin F)".
- ^ Hansson MJ, Morota S, Chen L, Matsuyama N, Suzuki Y, Nakajima S, Tanoue T, Omi A, Shibasaki F, Shimazu M, Ikeda Y, Uchino H, Elmér E (Jan 2011). "Cyclophilin D-sensitive mitochondrial permeability transition in adult human brain and liver mitochondria". Journal of Neurotrauma. 28 (1): 143–53. doi:10.1089/neu.2010.1613. PMC 3025768. PMID 21121808.
- ^ Kazui T, Inoue N, Yamada O, Komatsu S (Jan 1992). "Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment". The Annals of Thoracic Surgery. 53 (1): 109–14. doi:10.1016/0003-4975(92)90767-x. PMID 1530810.
- ^ Yao Q, Li M, Yang H, Chai H, Fisher W, Chen C (Mar 2005). "Roles of cyclophilins in cancers and other organ systems". World Journal of Surgery. 29 (3): 276–80. doi:10.1007/s00268-004-7812-7. PMID 15706440. S2CID 11678319.
- ^ Wang T, Yun CH, Gu SY, Chang WR, Liang DC (Aug 2005). "1.88 A crystal structure of the C domain of hCyP33: a novel domain of peptidyl-prolyl cis-trans isomerase". Biochemical and Biophysical Research Communications. 333 (3): 845–9. doi:10.1016/j.bbrc.2005.06.006. PMID 15963461.
- Stocki P, Chapman DC, Beach LA, Williams DB (Aug 2014). "Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis". The Journal of Biological Chemistry. 289 (33): 23086–96. doi:10.1074/jbc.M114.570911. PMC 4132807. PMID 24990953.
- Jandova J, Janda J, Sligh JE (Mar 2013). "Cyclophilin 40 alters UVA-induced apoptosis and mitochondrial ROS generation in keratinocytes". Experimental Cell Research. 319 (5): 750–60. doi:10.1016/j.yexcr.2012.11.016. PMC 3577976. PMID 23220213.
- Hoffmann H, Schiene-Fischer C (Jul 2014). "Functional aspects of extracellular cyclophilins". Biological Chemistry. 395 (7–8): 721–35. doi:10.1515/hsz-2014-0125. PMID 24713575. S2CID 32395688.
- ^ McStay GP, Clarke SJ, Halestrap AP (Oct 2002). "Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore". The Biochemical Journal. 367 (Pt 2): 541–8. doi:10.1042/BJ20011672. PMC 1222909. PMID 12149099.
- ^ Bowles KR, Zintz C, Abraham SE, Brandon L, Bowles NE, Towbin JA (Dec 1999). "Genomic characterization of the human peptidyl-prolyl-cis-trans-isomerase, mitochondrial precursor gene: assessment of its role in familial dilated cardiomyopathy". Human Genetics. 105 (6): 582–6. doi:10.1007/s004390051149. PMID 10647893.
- ^ He Y, Ge J, Tombran-Tink J (Nov 2008). "Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells". Investigative Ophthalmology & Visual Science. 49 (11): 4912–22. doi:10.1167/iovs.08-2192. PMID 18614807.
Further reading
- Crompton M, Virji S, Ward JM (Dec 1998). "Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore". European Journal of Biochemistry. 258 (2): 729–35. doi:10.1046/j.1432-1327.1998.2580729.x. PMID 9874241.
- Bowles KR, Zintz C, Abraham SE, Brandon L, Bowles NE, Towbin JA (Dec 1999). "Genomic characterization of the human peptidyl-prolyl-cis-trans-isomerase, mitochondrial precursor gene: assessment of its role in familial dilated cardiomyopathy". Human Genetics. 105 (6): 582–6. doi:10.1007/s004390051149. PMID 10647893.
- Bowles KR, Abraham SE, Brugada R, Zintz C, Comeaux J, Sorajja D, Tsubata S, Li H, Brandon L, Gibbs RA, Scherer SE, Bowles NE, Towbin JA (Jul 2000). "Construction of a high-resolution physical map of the chromosome 10q22-q23 dilated cardiomyopathy locus and analysis of candidate genes". Genomics. 67 (2): 109–27. doi:10.1006/geno.2000.6242. PMID 10903836.
- Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI (Jan 2002). "Directed proteomic analysis of the human nucleolus". Current Biology. 12 (1): 1–11. Bibcode:2002CBio...12....1A. doi:10.1016/S0960-9822(01)00650-9. PMID 11790298. S2CID 14132033.
- Lin DT, Lechleiter JD (Aug 2002). "Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization". The Journal of Biological Chemistry. 277 (34): 31134–41. doi:10.1074/jbc.M112035200. PMID 12077116.
- Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie MH, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A (Oct 2003). "The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment". Genome Research. 13 (10): 2265–70. doi:10.1101/gr.1293003. PMC 403697. PMID 12975309.
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M (Jan 2005). "Nucleolar proteome dynamics". Nature. 433 (7021): 77–83. Bibcode:2005Natur.433...77A. doi:10.1038/nature03207. PMID 15635413. S2CID 4344740.
External links
- PPIF human gene location in the UCSC Genome Browser.
- PPIF human gene details in the UCSC Genome Browser.
| PDB gallery | |
|---|---|