Medical intervention
| Radiofrequency ablation | |
|---|---|
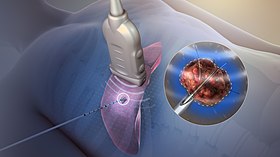 Tissue ablation using radiofrequency. Tissue ablation using radiofrequency. | |
| Specialty | Interventional radiology |
| ICD-9-CM | 01.32, 04.2, 37.33, 37.34, 60.97 |
| MeSH | D017115 |
| [edit on Wikidata] | |
Radiofrequency ablation (RFA), also called fulguration, is a medical procedure in which part of the electrical conduction system of the heart, tumor, sensory nerves or a dysfunctional tissue is ablated using the heat generated from medium frequency alternating current (in the range of 350–500 kHz). RFA is generally conducted in the outpatient setting, using either a local anesthetic or twilight anesthesia. When it is delivered via catheter, it is called radiofrequency catheter ablation.
Two advantages of radio frequency current (over previously used low frequency AC or pulses of DC) are that it does not directly stimulate nerves or heart muscle, and therefore can often be used without the need for general anesthesia, and that it is specific for treating the desired tissue without significant collateral damage. Due to this, RFA is an alternative for eligible patients who have comorbities or do not want to undergo surgery.
Documented benefits have led to RFA becoming widely used during the 21st century. RFA procedures are performed under image guidance (such as X-ray screening, CT scan or ultrasound) by an interventional pain specialist (such as an anesthesiologist), interventional radiologist, otolaryngologists, a gastrointestinal or surgical endoscopist, or a cardiac electrophysiologist, a subspecialty of cardiologists.
Tumors

RFA may be performed to treat tumors in the lung, liver, kidney, and bone, as well as other body organs less commonly. Once the diagnosis of tumor is confirmed, a needle-like RFA probe is placed inside the tumor. The radiofrequency waves passing through the probe increase the temperature within tumor tissue, which results in destruction of the tumor. RFA can be used with small tumors, whether these arose within the organ (primary tumors) or spread to the organ (metastases). The suitability of RFA for a particular tumor depends on multiple factors.
RFA can usually be administered as an outpatient procedure, though may at times require a brief hospital stay. RFA may be combined with locally delivered chemotherapy to treat hepatocellular carcinoma (primary liver cancer). A method currently in phase III trials uses the low-level heat (hyperthermia) created by the RFA probe to trigger release of concentrated chemotherapeutic drugs from heat-sensitive liposomes in the margins around the ablated tissue as a treatment for hepatocellular carcinoma (HCC). Radiofrequency ablation is also used in pancreatic cancer and bile duct cancer.
RFA has become increasingly important in the care of benign bone tumors, most notably osteoid osteomas. Since the procedure was first introduced for the treatment of osteoid osteomas in the 1990s, it has been shown in numerous studies to be less invasive and expensive, to result in less bone destruction and to have equivalent safety and efficacy to surgical techniques, with 66 to 95% of people reporting freedom from symptoms. While initial success rates with RFA are high, symptom recurrence after RFA treatment has been reported, with some studies demonstrating a recurrence rate similar to that of surgical treatment. RFA is also increasingly used in the palliative treatment of painful metastatic bone disease in people who are not eligible or do not respond to traditional therapies ( i.e. radiation therapy, chemotherapy, palliative surgery, bisphosphonates or analgesic medications).
Cardiology
See also: Catheter ablation
Radiofrequency energy is used in heart tissue or normal parts to destroy abnormal electrical pathways that are contributing to a cardiac arrhythmia. It is used in recurrent atrial flutter (Afl), atrial fibrillation (AF), supraventricular tachycardia (SVT), atrial tachycardia, Multifocal Atrial Tachycardia (MAT) and some types of ventricular arrhythmia. The energy-emitting probe (electrode) is at the tip of a catheter which is placed into the heart, usually through a vein. This catheter is called the ablator. The practitioner first "maps" an area of the heart to locate the abnormal electrical activity (electrophysiology study) before the responsible tissue is eliminated. Radiofrequency ablation technique can be used in AF, either to block the atrioventricular node after implantation of a pacemaker or to block conduction within the left atrium, especially around the pulmonary veins. Radiofrequency ablation for AF can be unipolar (one electrode) or bipolar (two electrodes). Although bipolar can be more successful, it is technically more difficult, resulting in unipolar being used more often. But bipolar is more effective in preventing recurrent atrial arrhythmias.
Ablation is now the standard treatment for SVT and typical atrial flutter, In some conditions, especially forms of intra-nodal re-entry (the most common type of SVT), also called atrioventricular nodal reentrant tachycardia or AVNRT, ablation can also be accomplished by cryoablation (tissue freezing using a coolant which flows through the catheter) which avoids the risk of complete heart block – a potential complication of radiofrequency ablation in this condition. Recurrence rates with cryoablation are higher, though. Microwave ablation, where tissue is ablated by the microwave energy "cooking" the adjacent tissue, and ultrasonic ablation, creating a heating effect by mechanical vibration, or laser ablation have also been developed but are not in widespread use.
Renal sympathetic denervation
See also: Renal sympathetic denervationA new indication for the use of radiofrequency technology has made news in the last few years. Hypertension is a very common condition, with about 1 billion people over the world, nearly 75 million in the US alone. Complications of inadequately controlled hypertension are many and have both individual and global impact. Treatment options include medications, diet, exercise, weight reduction and meditation. Inhibition of the neural impulses that are believed to cause or worsen hypertension has been tried for a few decades. Surgical sympathectomy has helped but not without significant side effects. Therefore, the introduction of non-surgical means of renal denervation using a radiofrequency ablation catheter was enthusiastically welcomed. Although the initial use of radiofrequency-generated heat to ablate nerve endings in the renal arteries to aid in management of 'resistant hypertension' were encouraging, the most recent phase 3 studying looking at catheter-based renal denervation for the treatment of resistant hypertension failed to show any significant reduction in systolic blood pressure.
Aesthetics dermatology
Radiofrequency ablation is a dermatosurgical procedure by using various forms of alternating current. Types of radiofrequency are electrosection, electrocoagulation, electrodessication and fulguration. The use of radiofrequency ablation has obtained importance as it can be used to treat most of the skin lesions with minimal side effects and complications.
Varicose veins
Radiofrequency ablation is a minimally invasive procedure used in the treatment of varicose veins. It is an alternative to the traditional stripping operation. Under ultrasound guidance, a radiofrequency catheter is inserted into the abnormal vein and the vessel treated with radio-energy, resulting in closure of the involved vein. Radiofrequency ablation is used to treat the great saphenous vein, the small saphenous vein, and the perforator veins. The latter are connecting veins that transport blood from the superficial veins to the deep veins. Branch varicose veins are then usually treated with other minimally invasive procedures, such as ambulatory phlebectomy, sclerotherapy, or foam sclerotherapy. Currently, the VNUS ClosureRFS stylet is the only device specifically cleared by FDA for endovenous ablation of perforator veins.
The possibility of skin burn during the procedure is very small, because the large volumes (500 cc) of dilute Lidocaine (0.1%) tumescent anesthesia injected along the entire vein prior to the application of radiofrequency provide a heat sink that absorbs the heat created by the device. Early studies have shown a high success rate with low rates of complications.
Obstructive sleep apnea
Main article: Obstructive sleep apnea § Radiofrequency ablationRFA was first studied in obstructive sleep apnea (OSA) in a pig model. RFA has been recognized as a somnoplasty treatment option in selected situations by the American Academy of Otolaryngology but was not endorsed for general use in the American College of Physicians guidelines.
The clinical application of RFA in obstructive sleep apnea is reviewed in that main article, including controversies and potential advantages in selected medical situations. Unlike other electrosurgical devices, RFA allows very specific treatment targeting of the desired tissue with a precise line of demarcation that avoids collateral damage, which is crucial in the head and neck region due to its high density of major nerves and blood vessels. RFA also does not require high temperatures. However, overheating from misapplication of RFA can cause harmful effects such as coagulation on the surface of the electrode, boiling within tissue that can leave "a gaping hole", tears, or even charring.
Pain management
Back
RFA, or rhizotomy, was developed by Nikolai Bogduk to treat chronic pain arising from the facet joints in the lower (lumbar) back. Radiofrequency waves are used to produce heat on specifically identified nerves surrounding the facet joints called the lumbar medial branches of the dorsal ramus of the spinal nerves. By generating heat around the nerve, the nerve is ablated, thus destroying its ability to transmit signals to the brain.
The nerves to be ablated are identified through injections of local anesthesia (such as lidocaine) around the medial branches prior to the RFA procedure to first confirm the diagnosis. If the local anesthesia injections provide temporary pain relief, the injection is repeated a second time to confirm the diagnosis. Then RFA is performed on the nerve(s) that responded well to the injections.
RFA is a minimally invasive procedure which can usually be done in day-surgery clinics, going home shortly after completion of the procedure. The person is awake during the procedure, so risks associated with general anesthesia are avoided. Whether for back or knee pain, a drawback for this procedure is that nerves recover function over time, so the pain relief achieved lasts only temporarily (3–15 months) in most people.
Knees
See also: Nerve block § Genicular nerve blockRadiofrequency ablation of sensory nerves in the knee, also called genicular neurotomy or genicular RFA, is clinically preceded by confirming pain reduction upon anesthetizing the main knee sensory nerves in a test procedure called genicular nerve block. Genicular nerve block is a short (10-30 minutes), outpatient procedure usually performed weeks before genicular RFA. The extent of pain reduction by injecting a local anesthetic, such as bupivacaine, at specific locations of the target genicular nerves, is self-assessed by the person for hours after the procedure, leading to confirmation with the physician of the need for RFA.
In the procedure for genicular RFA, a guide cannula is first directed under local anesthesia and imaging (ultrasound or fluoroscopy) to each target genicular nerve, then the radiofrequency electrode is passed through the cannula, and the electrode tip is heated to about 80 °C (176 °F) for one minute to cauterize a small segment of the nerve. The heat destroys that segment of the nerve, which is prevented from sending pain signals to the brain.
As of 2019, several hundred publications showed promise for substantial, long-term (6 months or longer) reduction of knee pain following genicular RFA.
The US Food and Drug Administration had approved in 2017 a commercial device using cooled RFA, with effects lasting for up to a year of pain relief from knee arthritis. As of 2023, reviews of clinical outcomes indicated that efficacy for reducing knee pain was achieved by ablating three or more branches of the genicular nerve (one of the articular branches of the tibial nerve). Other sources indicate 4-5 genicular nerve targets may be justified for ablation to optimize pain relief, while a 2022 analysis indicated that as many as 10 genicular nerve targets for RFA would produce better long-term relief of knee pain.
Knee pain relief of 50% or more following genicular RFA may last from several months to two years, and can be repeated by the same outpatient procedure when pain recurs.
An anatomical study of cadaver knees indicated that ultrasound-guided bony landmarks could be used to effectively target the superior medial geniculate nerve, superior lateral geniculate nerve, and inferior medial geniculate nerve – the three nerves commonly targeted for knee RFA – with average nerve-to-needle distances of 1.7, 3.2, and 1.8 mm, respectively.
Barrett's esophagus
Radiofrequency ablation has been shown to be a safe and effective treatment for Barrett's esophagus. The balloon-based radiofrequency procedure was invented by Robert A. Ganz, Roger Stern and Brian Zelickson in 1999 (System and Method for Treating Abnormal Tissue in the Human Esophagus). While the person is sedated, a catheter is inserted into the esophagus and radiofrequency energy is delivered to the diseased tissue. This outpatient procedure typically lasts from fifteen to thirty minutes. Two months after the procedure, the physician performs an upper endoscopic examination to assess the esophagus for residual Barrett's esophagus. If any Barrett's esophagus is found, the disease can be treated with a focal RFA device. Between 80 and 90% or greater of people in numerous clinical trials have shown complete eradication of Barrett's esophagus in approximately two to three treatments with a favorable safety profile. The treatment of Barrett's esophagus by RFA is durable for up to 5 years.
Thyroid nodules
Radiofrequency ablation has been used successfully on benign thyroid nodules for decades, most notably in Europe, South America and Korea. In the United States, the FDA approved the use of RFA techniques for thyroid nodules in 2018. Since then, professional guidelines reflect its use as a viable treatment modality for thyroid nodules, and the procedure is increasingly applied.
Timeline in the United States
- 2023: the American Thyroid Association issued the position statement "Thyroid ablative procedures provide valid alternative treatment strategies to conventional surgical management for a subset of patients with symptomatic benign thyroid nodules.
- 2022: the American Association of Clinical Endocrinologists published an update in Endocrine Practice, stating that the "new image-guided minimally invasive approaches appear safe and effective alternatives when used appropriately and by trained professionals to treat symptomatic or enlarging thyroid masses".
- 2018: FDA approved the RFA procedure for treatment of benign thyroid nodules.
Procedure
The procedure is similar to a thyroid biopsy, although instead of using a needle to remove cells from the nodule, a probe delivers heat to the interior of the nodule that effectively cauterizess the tissue. Over the course of 3-6 months, the nodule will continue to shrink, typically achieving a 50-80% reduction total size.
In order to qualify for an RFA procedure, a person must have a clearly benign thyroid nodule, usually proven by two fine needle aspiration biopsies.
As of 2020, RFA is not recommended for the treatment of malignant thyroid nodules, although research into this topic is ongoing.
Other uses
RFA is also used in radiofrequency lesioning for vein closure in areas where intrusive surgery is contraindicated by trauma, and in liver resection to control bleeding (hemostasis) and facilitate the transection process.
This process has also been used to treat TRAP sequence in multiple gestation pregnancies. This has an acceptable success rate for saving the 'pump' twin in recent studies compared to previous methods including laser photocoagulation.
RFA is used to treat uterine fibroids using the heat energy of radio frequency waves to ablate the fibroid tissue. The Acessa device obtained FDA approval in 2012. The device is inserted via a laparoscopic probe and guided inside the fibroid tissue using an ultrasound probe. The heat shrinks the fibroids. Clinical data on the procedure show an average of 45% shrinkage.
RFA is also used in the treatment of Morton's neuroma where the outcome appears to be more reliable than alcohol injections.
See also
References
- "Fulguration: NCI Dictionary of Cancer Terms". National Cancer Institute. February 2, 2011. Retrieved May 10, 2018.
- Courtney M. Townsend (2012). Sabiston textbook of surgery : the biological basis of modern surgical practice (19th ed.). Philadelphia, PA: Elsevier Saunders. p. 236. ISBN 978-1-4377-1560-6.
- ^ Kidd VD, Strum SR, Strum DS, et al. (March 2019). "Genicular Nerve Radiofrequency Ablation for Painful Knee Arthritis: The Why and the How". JBJS Essential Surgical Techniques. 9 (1): e10. doi:10.2106/JBJS.ST.18.00016. PMC 6635137. PMID 31333900.
- "Radiofrequency neurotomy". Mayo Clinic. April 6, 2024. Retrieved September 5, 2024.
- ^ "Radiofrequency Ablation for Pain Management". Cleveland Clinic. March 14, 2022. Retrieved September 5, 2024.
- Hussain I, Zulfiqar F, Li X, et al. (August 1, 2021). "Safety and Efficacy of Radiofrequency Ablation of Thyroid Nodules-Expanding Treatment Options in the United States". Journal of the Endocrine Society. 5 (8): bvab110. doi:10.1210/jendso/bvab110. ISSN 2472-1972. PMC 8271212. PMID 34258495.
- "Ablation for Arrhythmias". American Heart Association. 2017.
- "Radiofrequency ablation for cancer". Mayo Clinic. 2017.
- ^ Dunn, Lauren, Gussone, Felix (June 13, 2017). "'Cool' New Knee Procedure Eases Arthritis Pain Without Surgery". NBC News, New York. Retrieved June 13, 2017.
- Ambrogi MC, Fanucchi O, Cioni R, et al. (2011). "Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study". J Thorac Oncol. 6 (12): 2044–51. doi:10.1097/JTO.0b013e31822d538d. PMID 22052222.
- "NHS, June 2008 – Radiofrequency ablation for lung cancer". Archived from the original on June 7, 2016. Retrieved May 29, 2010.
- Daily Telegraph – June 2008 – Lung cancer radiation treatment offers new hope
- BBC News- 16 January 2009 – Liver tumours 'microwaved away'
- Phase 3 Study of ThermoDox With Radiofrequency Ablation (RFA) in Treatment of Hepatocellular Carcinoma (HCC)
- Hadjicostas P, Malakounides N, Varianos C, et al. (2006). "Radiofrequency ablation in pancreatic cancer". HPB. 8 (1): 61–64. doi:10.1080/13651820500466673. PMC 2131369. PMID 18333241.
- Rosenthal DI, Alexander A, Rosenberg AE, et al. (April 1, 1992). "Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure". Radiology. 183 (1): 29–33. doi:10.1148/radiology.183.1.1549690. ISSN 0033-8419. PMID 1549690.
- Weber MA, Sprengel SD, Omlor GW, et al. (April 25, 2015). "Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection". Skeletal Radiology. 44 (7): 981–93. doi:10.1007/s00256-015-2139-z. ISSN 0364-2348. PMID 25910709. S2CID 21496405.
- Rosenthal DI, Hornicek FJ, Torriani M, et al. (October 1, 2003). "Osteoid Osteoma: Percutaneous Treatment with Radiofrequency Energy". Radiology. 229 (1): 171–75. doi:10.1148/radiol.2291021053. ISSN 0033-8419. PMID 12944597.
- Rimondi E, Mavrogenis AF, Rossi G, et al. (August 14, 2011). "Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients". European Radiology. 22 (1): 181–88. doi:10.1007/s00330-011-2240-1. ISSN 0938-7994. PMID 21842430. S2CID 21047698.
- Rosenthal DI, Hornicek FJ, Wolfe MW, et al. (June 1, 1998). "Percutaneous Radiofrequency Coagulation of Osteoid Osteoma Compared with Operative Treatment*". J Bone Joint Surg Am. 80 (6): 815–21. CiteSeerX 10.1.1.1018.5024. doi:10.2106/00004623-199806000-00005. ISSN 0021-9355. PMID 9655099. S2CID 10709128. Archived from the original on October 6, 2016. Retrieved August 7, 2016.
- Dupuy DE, Liu D, Hartfeil D, et al. (February 15, 2010). "Percutaneous radiofrequency ablation of painful osseous metastases". Cancer. 116 (4): 989–97. doi:10.1002/cncr.24837. ISSN 1097-0142. PMC 2819592. PMID 20041484.
- ^ Soucek F, Starek Z (2018). "Use of Bipolar Radiofrequency Catheter Ablation in the Treatment of Cardiac Arrhythmias". Current Cardiology Reviews. 14 (3): 185–191. doi:10.2174/1573403X14666180524100608. PMC 6131405. PMID 29792146.
- Pearman CM, Poon SS, Gupta D (2017). "Minimally Invasive Epicardial Surgical Ablation Alone Versus Hybrid Ablation for Atrial Fibrillation: A Systematic Review and Meta-Analysis". Arrhythmia & Electrophysiology Review. 6 (4): 202–209. doi:10.15420/aer/2017.29.2. PMC 5739900. PMID 29326836.
- Deisenhofer I, Zrenner B, Yin YH, et al. (2010). "Cryoablation Versus Radiofrequency Energy for the Ablation of Atrioventricular Nodal Reentrant Tachycardia (the CYRANO Study) : Results From a Large Multicenter Prospective Randomized Trial". Circulation. 122 (22): 2239–45. doi:10.1161/circulationaha.110.970350. PMID 21098435.
- Bhatt DL, Kandzari KE, O'Neill WW, et al. (2014). "A Controlled Trial of Renal Denervation for Resistant Hypertension (SYMPLICITY HTN-3 Trial)". N Engl J Med. 370 (15): 1393–401. doi:10.1056/NEJMoa1402670. PMID 24678939. S2CID 3653990.
- Loesch MM, Somani AK, Kingsley MM, et al. (2014). "Skin resurfacing procedures: new and emerging options". Clin Cosmet Investig Dermatol. 7: 231–41. doi:10.2147/CCID.S50367. PMC 4155739. PMID 25210469.
- Endovenous ablation of perforator veins
- Avery J, Kumar K, Thakur V, et al. (2014). "Radiofrequency ablation as first-line treatment of varicose veins". Am Surg. 80 (3): 231–35. doi:10.1177/000313481408000316. PMID 24666862. S2CID 23552772.
- ^ "Submucosal Ablation of the Tongue Base for OSAS". American Academy of Otolaryngology – Head and Neck Surgery. Retrieved October 29, 2013.
- Qaseem A, Holty, JE, Owens, DK, et al. (September 24, 2013). "Management of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 159 (7): 471–83. doi:10.7326/0003-4819-159-7-201310010-00704. PMID 24061345.
- Bashetty K, Gururaj Nadig Sandhya Kapoor (November 19, 2009). "Electrosurgery in aesthetic and restorative dentistry: A literature review and case reports". Journal of Conservative Dentistry. 12 (4): 139–44. doi:10.4103/0972-0707.58332. PMC 2879725. PMID 20543922.
- Eick OJ (July 1, 2002). "Temperature Controlled Radiofrequency Ablation". Indian Pacing Electrophysiol. 3. 2 (3): 66–73. PMC 1564057. PMID 17006561.
- Russo M, Santarelli D, Wright R, et al. (2021). "A History of the Development of Radiofrequency Neurotomy". Journal of Pain Research. 14: 3897–3907. doi:10.2147/JPR.S334862. ISSN 1178-7090. PMC 8714970. PMID 34992451.
- ^ "Radiofrequency ablation for pain management". Cleveland Clinic. March 14, 2022. Retrieved September 6, 2024.
- ^ Tran A, Reiter DA, Cruz AR, et al. (April 2022). "Genicular Nerve Ablation Review Using Cooled-Radiofrequency Nerve Ablation". Seminars in Interventional Radiology. 39 (2): 130–137. doi:10.1055/s-0042-1745797. PMC 9246497. PMID 35781999.
- ^ Conger A, Gililland J, Anderson L, et al. (July 2021). "Genicular Nerve Radiofrequency Ablation for the Treatment of Painful Knee Osteoarthritis: Current Evidence and Future Directions". Pain Medicine. 22 (Suppl 1): S20–S23. doi:10.1093/pm/pnab129. PMID 34308957.
- ^ "Genicular nerve block". Cleveland Clinic. March 17, 2023. Retrieved September 18, 2024.
- ^ Ariel de Lima D, Gonçalves MC, Grando ST, et al. (May 2019). "Indications of the Neurotomy of Genicular Nerves by Radiofrequency for the Treatment of Knee Osteoarthritis: A Literature Review". Revista Brasileira De Ortopedia. 54 (3): 233–240. doi:10.1055/s-0039-1692121. PMC 6597460. PMID 31363275.
- Jessup, Cynthia (April 21, 2017). "FDA Green Lights Halyard Health's Coolief for the Management of Osteoarthritis Knee Pain". FDA News. Retrieved June 13, 2017.
- ^ Tran A, Gonzalez FM (2023). "Review of cooled radiofrequency ablation utilization for the treatment of symptomatic advanced knee arthritis and total knee arthroplasty". Skeletal Radiology. 52 (5): 941–949. doi:10.1007/s00256-022-04058-w. PMID 35462577.
- ^ Gupta A, Huettner DP, Dukewich M (2017). "Comparative Effectiveness Review of Cooled Versus Pulsed Radiofrequency Ablation for the Treatment of Knee Osteoarthritis: A Systematic Review". Pain Physician. 20 (3): 155–171. doi:10.36076/ppj.2017.171. PMID 28339430.
- Greco G, Torres J, Kohan L (2022). "How I Do It: Genicular Nerve Radiofrequency Ablation". ASRA Pain Medicine News, #47. doi:10.52211/asra020122.006.
- Kolakkanni C, Gonnade NM, Gaur R, et al. (August 2023). "Can ultrasound-guided radiofrequency ablation of genicular nerves of the knee, be performed without locating corresponding arterial pulsations – a cadaveric study". BMC Musculoskeletal Disorders. 24 (1): 654. doi:10.1186/s12891-023-06761-8. PMC 10429091. PMID 37587439.
- Ganz R, Utley D, Stern R, et al. (2004). "Complete Ablation of Esophageal Epithelium Using a Balloon-based Bipolar Electrode". Gastrointestinal Endoscopy. 60 (6): 1002–10. doi:10.1016/s0016-5107(04)02220-5. PMID 15605025.
- Fleischer DE, Overholt BF, Sharma VK, et al. (2010). "Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial". Endoscopy. 42 (10): 781–89. doi:10.1055/s-0030-1255779. PMID 20857372. S2CID 24224546.
- Shaheen NJ, Sharma P, Overholt BF, et al. (2009). "Radiofrequency Ablation in Barrett's Esophagus with Dysplasia". New England Journal of Medicine. 360 (22): 2277–88. doi:10.1056/NEJMoa0808145. PMID 19474425. S2CID 22864452.
- Shaheen NJ, Overholt BF, Sampliner RE, et al. (2011). "Durability of Ablation in Barrett's Esophagus with Dysplasia". Gastroenterology. 141 (2): 460–68. doi:10.1053/j.gastro.2011.04.061. PMC 3152658. PMID 21679712.
- Van Vilsteren FG, Pouw RE, Seewald S, et al. (June 2011). "Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high grade dysplasia or early cancer: a multicentre randomised trial". Gut. 60 (6): 765–73. doi:10.1136/gut.2010.229310. PMID 21209124. S2CID 53530205.
- Sinclair CF, Baek JH, Hands KE, et al. (October 2023). "General Principles for the Safe Performance, Training, and Adoption of Ablation Techniques for Benign Thyroid Nodules: An American Thyroid Association Statement". Thyroid. 33 (10): 1150–1170. doi:10.1089/thy.2023.0281. PMC 10611977. PMID 37642289.
- Jasim S, Patel KN, Randolph G, et al. (April 2022). "American Association of Clinical Endocrinology Disease State Clinical Review: The Clinical Utility of Minimally Invasive Interventional Procedures in the Management of Benign and Malignant Thyroid Lesions". Endocrine Practice. 28 (4): 433–448. doi:10.1016/j.eprac.2022.02.011. PMID 35396078.
- Rangel LG, Steck JH, Volpi EM, et al. (2022). "Radiofrequency Ablation of Papillary Thyroid Microcarcinomas". AACE Clinical Case Reports. 8 (2): 99–101. doi:10.1016/j.aace.2022.02.005. PMC 8984508. PMID 35415224.
- "Acessa Health Procedure".
- King, Paula (December 10, 2012). "Brentwood medical company obtains FDA approval for new medical device". San Jose, CA: The Mercury News, Digital First Media.
- Chuter GS, Chua YP, Connell DA, et al. (January 2013). "Ultrasound-guided radiofrequency ablation in the management of interdigital (Morton's) neuroma". Skeletal Radiol. 42 (1): 107–11. doi:10.1007/s00256-012-1527-x. PMID 23073898. S2CID 25166343.
- Gurdezi S, White T, Ramesh P (2013). "Alcohol injection for Morton's neuroma: a five-year follow-up". Foot Ankle Int. 34 (8): 1064–67. doi:10.1177/1071100713489555. PMID 23669161. S2CID 7317044.