| |
| Names | |
|---|---|
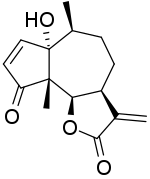
| IUPAC name 1-Hydroxy-6β,12-epoxyambrosa-2,11(13)-diene-4,12-dione | |
| Systematic IUPAC name (3aS,6S,6aS,9aS,9bR)-6a-Hydroxy-6,9a-dimethyl-3-methylidene-3,3a,4,5,6,6a,9a,9b-octahydroazulenofuran-2,9-dione | |
| Other names Parthenicin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H18O4 |
| Molar mass | 262.305 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Parthenin is a chemical compound classified as a sesquiterpene lactone. It has been isolated from Parthenium hysterophorus.
It is genotoxic, allergenic, and an irritant. Parthenin is believed to be responsible for the dermatitis caused by Parthenium hysterophorus.
References
- Ramos, Alberto; Rivero, Reinaldo; Visozo, Angel; Piloto, Janet; Garcı́a, Arilia (2002). "Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. Is a high toxicity clastogen". Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 514 (1–2): 19–27. doi:10.1016/S1383-5718(01)00321-7. PMID 11815241.
- Picman, J.; Picman, A. K. (1985). "Treatment of dermatitis from parthenin". Contact Dermatitis. 13 (1): 9–13. doi:10.1111/j.1600-0536.1985.tb02484.x. PMID 4042647. S2CID 40846706.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |