| |

| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.022.363 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
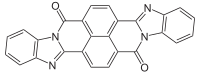
| Chemical formula | C26H12N4O2 |
| Molar mass | 412.408 g·mol |
| Appearance | Orange solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Perinone is a class of organic compounds. The parent compound has two isomers, each of which are useful pigments.
It is prepared from naphthalenetetracarboxylic dianhydride by condensation with o-phenylenediamine. The two Isomers of perinone are useful pigments. The trans isomer is called Pigment Orange 43 ("PO43", CID 78141 from PubChem) and the cis isomer is called Pigment Red 194 ("PR194", CID 77892 from PubChem). Like some structurally related compounds perinone is also an organic semiconductor.
References
- Mizuguchi, Jin (2004). "Crystal Structure and Electronic Characterization of trans-and cis-Perinone Pigments". J. Phys. Chem. B. 108 (26): 8926–8930. doi:10.1021/jp031351d.
- K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- Feast, W. James; Peace, Richard J.; Sage, Ian C.; Wood, Emma L. (March 1999). "Poly(4-vinyltriphenylamine): synthesis and application as a hole transport layer in light-emitting diodes". Polymer Bulletin. 42 (2): 167–174. doi:10.1007/s002890050449. S2CID 95994481.