| |
| |
| Names | |
|---|---|
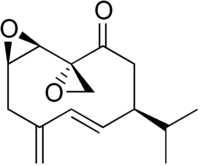
| IUPAC name (5E)-1β,2β:10,14-Diepoxygermacra-4(15),5-dien-9-one | |
| Systematic IUPAC name (1R,2R,5S,6E,10R)-8-Methylidene-5-(propan-2-yl)-11-oxaspiroundecane-2,2′-oxiran]-6-en-3-one | |
| Other names (1Z,5E)-1,10(14)-Diepoxy-4(15),5-germacradiene-9-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
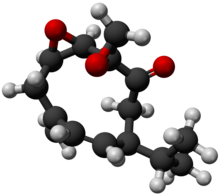
| Chemical formula | C15H20O3 |
| Molar mass | 248.322 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Periplanone B is a pheromone produced by the female American cockroach, Periplaneta americana. It is a sexual attractant to male cockroaches, especially at short ranges.
History
The activity of this pheromone was first described in 1952, but it was not until 25 years later that Persoons et al. reported the gross structure of periplanones A and B. The stereochemical configuration and first total synthesis were reported by W. Clark Still's group at Columbia University in 1979.
References
- Okada, K; et al. (September 1990). "Behavioral responses of male Periplaneta americana L. to female sex pheromone components, periplanone-A and periplanone-B". Journal of Chemical Ecology. 16 (9): 2605–2614. doi:10.1007/BF00988072. PMID 24264316. S2CID 30323914.
- Chow, YF; Wang, SF (1981). "Attraction responses of the American cockroach to synthetic periplanone-B". Journal of Chemical Ecology. 7 (2): 265–272. doi:10.1007/BF00995749. PMID 24420472. S2CID 21794952.
- Nicolaou, K. C.; E. J. Sorensen (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. p. 211. ISBN 3-527-29284-5.
This article about a ketone is a stub. You can help Misplaced Pages by expanding it. |