The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the periplasmic space in Gram-negative (more accurately "diderm") bacteria. Using cryo-electron microscopy it has been found that a much smaller periplasmic space is also present in Gram-positive bacteria (more accurately "monoderm"), between cell wall and the plasma membrane. The periplasm may constitute up to 40% of the total cell volume of gram-negative bacteria, but is a much smaller percentage in gram-positive bacteria.
Terminology
Although bacteria are conventionally divided into two main groups—Gram-positive and Gram-negative, based upon their Gram-stain retention property—this classification system is ambiguous as it can refer to three distinct aspects (staining result, cell-envelope organization, taxonomic group), which do not necessarily coalesce for some bacterial species. In most situations such as in this article, Gram-staining reflects the marked differences in the ultrastructure and chemical composition of the two main kinds of bacteria. The usual "Gram-positive" type does not have an outer lipid membrane, while the typical "Gram-negative" bacterium does. The terms "diderm" and "monoderm", coined to refer to this distinction only, is a more reliable and fundamental characteristic of the bacterial cells.
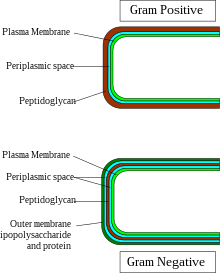
All Gram-positive bacteria are bounded by a single unit lipid membrane (i.e. monoderm); they generally contain a thick layer (20-80 nm) of peptidoglycan responsible for retaining the Gram-stain. A number of other bacteria which are bounded by a single membrane but stain gram-negative due to either lack of the peptidoglycan layer (viz., mycoplasmas) or their inability to retain the Gram-stain due to their cell wall composition, also show close relationship to the Gram-positive bacteria. For the bacterial (prokaryotic) cells that are bounded by a single cell membrane the term "monoderm bacteria" or "monoderm prokaryotes" has been proposed. In contrast to gram-positive bacteria, all archetypical Gram-negative bacteria are bounded by a cytoplasmic membrane as well as an outer cell membrane; they contain only a thin layer of peptidoglycan (2–3 nm) between these membranes. The presence of both inner and outer cell membranes forms and define the periplasmic space or periplasmic compartment. These bacterial cells with two membranes have been designated as diderm bacteria. The distinction between the monoderm and diderm prokaryotes is supported by conserved signature indels in a number of important proteins (for example, DnaK and GroEL).
Structure

As shown in the figure to the right, the periplasmic space in gram-negative or diderm bacteria is located between the inner and outer membrane of the cell. The periplasm contains peptidoglycan and the membranes that enclose the periplasmic space contain many integral membrane proteins, which can participate in cell signaling. Furthermore, the periplasm houses motility organelles such as the flagellum, which spans both membranes enclosing the periplasm. The periplasm is described as gel-like due to the high abundance of proteins and peptidoglycan. The periplasm occupies 7% to 40% of the total volume of diderm bacteria, and contains up to 30% of cellular proteins. The structure of the monoderm periplasm differs from that of diderm bacteria as the so-called periplasmic space in monoderm bacteria is not enclosed by two membranes but is rather enclosed by the cytoplasmic membrane and the peptidoglycan layer beneath. For this reason, the monoderm periplasmic space is also referred to as the inner-wall zone (IWZ). The IWZ serves as the first destination of translocation for proteins being transported across the monoderm bacterial cell wall.
Function
In diderm bacteria, the periplasm contains a thin cell wall composed of peptidoglycan. In addition, it includes solutes such as ions and proteins, which are involved in wide variety of functions ranging from nutrient binding, transport, folding, degradation, substrate hydrolysis, to peptidoglycan synthesis, electron transport, and alteration of substances toxic to the cell (xenobiotic metabolism). Importantly, the periplasm is devoid of ATP. Several types of enzyme are present in the periplasm including alkaline phosphatases, cyclic phosphodiesterases, acid phosphatases and 5’-nucleotidases. Of note, the periplasm also contains enzymes important for the facilitation of protein folding. For example, disulfide bond protein A (DsbA) and disulfide bond protein C (DsbC), which are responsible for catalyzing peptide bond formation and isomerization, respectively, were identified in the periplasm of E. Coli. As disulfide bond formation is frequently a rate-limiting step in the folding of proteins, these oxidizing enzymes play an important role in the bacteria periplasm. In addition, the periplasm mediates the uptake of DNA in several strains of transformable bacteria.

The compartmentalization afforded by the periplasmic space gives rise to several important functions. Aside from those previously mentioned, the periplasm also functions in protein transport and quality control, analogous to the endoplasmic reticulum in eukaryotes. Furthermore, the separation of the periplasm from the cytoplasm allows for the compartmentalization of enzymes that could be toxic in the cytoplasm. Some peptidoglycans and lipoproteins located in the periplasm provide a structural support system for the cell that aids in promoting the cell's ability to withstand turgor pressure. Notably, organelles such as the flagellum require the assembly of polymers within the periplasm for proper functioning. As the driveshaft of the flagellum spans the periplasmic space, its length is dictated by positioning of the outer membrane as induced by its contraction, which is mediated by periplasmic polymers. The periplasm also functions in cell signaling, such as in the case of the lipoprotein RcsF, which has a globular domain residing in the periplasm and acts as a stress sensor. When RcsF fails to interact with BamA, such as in the case of an enlarged periplasm, RcsF is not exported to the cell surface and are able to trigger the Rcs signaling cascade. Periplasm size, therefore, plays an important role in stress signaling.
Clinical significance
As bacteria are the responsible pathogen for many infections and illnesses, the biochemical and structural components that distinguish disease causing bacterial cells from native eukaryotic cells are of great interest from a clinical perspective. Gram-negative bacteria tend to be more antimicrobial resistant than gram-positive bacteria, and also possess a much more significant periplasmic space between their two membrane bilayers. Since eukaryotes do not possess a periplasmic space, structures and enzymes found in the gram-negative periplasm are attractive targets for antimicrobial drug therapies. Additionally, vital functions such as facilitation of protein folding, protein transport, cell signaling, structural integrity, and nutrient uptake are performed by periplasm components, making it rich in potential drug targets. Aside from enzymes and structural components that are vital to cell function and survival, the periplasm also contains virulence-associated proteins such as DsbA that can be targeted by antimicrobial therapies. Due to their role in catalyzing disulfide bond formation for a variety of virulence factors, the DsbA/DsbB system has been of particular interest as a target for anti-virulence drugs.
The periplasmic space is deeply interconnected with the pathogenesis of disease in the setting of microbial infection. Many of the virulence factors associated with bacterial pathogenicity are secretion proteins, which are often subject to post-translational modification including disulfide bond formation. The oxidative environment of the periplasm contains Dsb (disulfide bond formation) proteins that catalyze such post-translational modifications, and therefore play an important role in establishing virulence factor tertiary and quaternary structure essential for proper protein function. In addition to Dsb proteins found in the periplasm, motility organelles such as the flagellum are also essential for host infection. The flagellum is rooted in the periplasm and is stabilized by interaction with periplasmic structural components, and is therefore another pathogenesis-related target for antimicrobial agents. During infection of a host, the cell of a bacterium is subject to many turbulent environmental conditions, which highlights the importance of the structural integrity afforded by the periplasm. In particular, peptidoglycan synthesis is vital to cell wall production, and inhibitors of peptidoglycan synthesis have been of clinical interest for targeting bacteria for many decades. Furthermore, the periplasm is also relevant to clinical developments by way of its role in mediating the uptake of transforming DNA.
References
- Matias VR, Beveridge TJ (April 2005). "Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space". Molecular Microbiology. 56 (1): 240–251. doi:10.1111/j.1365-2958.2005.04535.x. PMID 15773993. S2CID 11013569.
- ^ Zuber B, Haenni M, Ribeiro T, Minnig K, Lopes F, Moreillon P, Dubochet J (September 2006). "Granular layer in the periplasmic space of gram-positive bacteria and fine structures of Enterococcus gallinarum and Streptococcus gordonii septa revealed by cryo-electron microscopy of vitreous sections". Journal of Bacteriology. 188 (18): 6652–6660. doi:10.1128/JB.00391-06. PMC 1595480. PMID 16952957.
- Holst O, Seltmann G (January 2002). The Bacterial Cell Wall. Berlin: Springer. ISBN 3-540-42608-6.
- ^ Gupta RS (December 1998). "Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes". Microbiology and Molecular Biology Reviews. 62 (4): 1435–1491. doi:10.1128/MMBR.62.4.1435-1491.1998. PMC 98952. PMID 9841678.
- ^ Gupta RS (2000). "The natural evolutionary relationships among prokaryotes". Critical Reviews in Microbiology. 26 (2): 111–131. doi:10.1080/10408410091154219. PMID 10890353. S2CID 30541897.
- Desvaux M, Hébraud M, Talon R, Henderson IR (April 2009). "Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue". Trends in Microbiology. 17 (4): 139–145. doi:10.1016/j.tim.2009.01.004. PMID 19299134.
- Sutcliffe IC (October 2010). "A phylum level perspective on bacterial cell envelope architecture". Trends in Microbiology. 18 (10): 464–470. doi:10.1016/j.tim.2010.06.005. PMID 20637628.
- ^ Gupta RS (August 1998). "What are archaebacteria: life's third domain or monoderm prokaryotes related to gram-positive bacteria? A new proposal for the classification of prokaryotic organisms". Molecular Microbiology. 29 (3): 695–707. doi:10.1046/j.1365-2958.1998.00978.x. PMID 9723910. S2CID 41206658.
- Gupta RS (August 2011). "Origin of diderm (Gram-negative) bacteria: antibiotic selection pressure rather than endosymbiosis likely led to the evolution of bacterial cells with two membranes". Antonie van Leeuwenhoek. 100 (2): 171–182. doi:10.1007/s10482-011-9616-8. PMC 3133647. PMID 21717204.
- Prochnow, Hans; Fetz, Verena; Hotop, Sven-Kevin; García-Rivera, Mariel A.; Heumann, Axel; Brönstrup, Mark (2019-02-05). "Subcellular Quantification of Uptake in Gram-Negative Bacteria". Analytical Chemistry. 91 (3): 1863–1872. doi:10.1021/acs.analchem.8b03586. hdl:10033/621709. ISSN 0003-2700.
- Weiner, Joel H.; Li, Liang (September 2008). "Proteome of the Escherichia coli envelope and technological challenges in membrane proteome analysis". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1778 (9): 1698–1713. doi:10.1016/j.bbamem.2007.07.020.
- ^ Forster, Brian M.; Marquis, Hélène (May 2012). "Protein transport across the cell wall of monoderm Gram‐positive bacteria". Molecular Microbiology. 84 (3): 405–413. doi:10.1111/j.1365-2958.2012.08040.x. ISSN 0950-382X. PMC 3331896. PMID 22471582.
- Klein DW, Prescott LM, Harley J (2005). Microbiology. Boston: McGraw-Hill Higher Education. ISBN 0-07-295175-3.
- Neu HC, Heppel LA (September 1965). "The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts". The Journal of Biological Chemistry. 240 (9): 3685–3692. doi:10.1016/S0021-9258(18)97200-5. PMID 4284300.
- Denoncin K, Collet JF (July 2013). "Disulfide bond formation in the bacterial periplasm: major achievements and challenges ahead". Antioxidants & Redox Signaling. 19 (1): 63–71. doi:10.1089/ars.2012.4864. PMC 3676657. PMID 22901060.
- ^ Hahn J, DeSantis M, Dubnau D (June 2021). Freitag NE (ed.). "Mechanisms of Transforming DNA Uptake to the Periplasm of Bacillus subtilis". mBio. 12 (3): e0106121. doi:10.1128/mBio.01061-21. PMC 8262900. PMID 34126763.
- ^ Miller SI, Salama NR (January 2018). "The gram-negative bacterial periplasm: Size matters". PLOS Biology. 16 (1): e2004935. doi:10.1371/journal.pbio.2004935. PMC 5771553. PMID 29342145.
- Rodríguez-Alonso R, Létoquart J, Nguyen VS, Louis G, Calabrese AN, Iorga BI, et al. (September 2020). "Structural insight into the formation of lipoprotein-β-barrel complexes". Nature Chemical Biology. 16 (9): 1019–1025. doi:10.1038/s41589-020-0575-0. PMC 7610366. PMID 32572278.
- Prestinaci F, Pezzotti P, Pantosti A (2015-10-03). "Antimicrobial resistance: a global multifaceted phenomenon". Pathogens and Global Health. 109 (7): 309–318. doi:10.1179/2047773215Y.0000000030. PMC 4768623. PMID 26343252.
- Pandeya A, Ojo I, Alegun O, Wei Y (September 2020). "Periplasmic Targets for the Development of Effective Antimicrobials against Gram-Negative Bacteria". ACS Infectious Diseases. 6 (9): 2337–2354. doi:10.1021/acsinfecdis.0c00384. PMC 8187054. PMID 32786281.
- Ha UH, Wang Y, Jin S (March 2003). "DsbA of Pseudomonas aeruginosa is essential for multiple virulence factors". Infection and Immunity. 71 (3): 1590–1595. doi:10.1128/IAI.71.3.1590-1595.2003. PMC 148828. PMID 12595484.
- Smith RP, Paxman JJ, Scanlon MJ, Heras B (July 2016). "Targeting Bacterial Dsb Proteins for the Development of Anti-Virulence Agents". Molecules. 21 (7): 811. doi:10.3390/molecules21070811. PMC 6273893. PMID 27438817.
- ^ Łasica AM, Jagusztyn-Krynicka EK (September 2007). "The role of Dsb proteins of Gram-negative bacteria in the process of pathogenesis". FEMS Microbiology Reviews. 31 (5): 626–636. doi:10.1111/j.1574-6976.2007.00081.x. PMID 17696887.
- Puls JS, Brajtenbach D, Schneider T, Kubitscheck U, Grein F (March 2023). "Inhibition of peptidoglycan synthesis is sufficient for total arrest of staphylococcal cell division". Science Advances. 9 (12): eade9023. Bibcode:2023SciA....9E9023P. doi:10.1126/sciadv.ade9023. PMC 10032595. PMID 36947615.
- Linnett PE, Strominger JL (September 1973). "Additional antibiotic inhibitors of peptidoglycan synthesis". Antimicrobial Agents and Chemotherapy. 4 (3): 231–236. doi:10.1128/AAC.4.3.231. PMC 444534. PMID 4202341.
Further reading
- White D (2000). The Physiology and Biochemistry of Prokaryotes (2nd ed.). Oxford: Oxford University Press. p. 22. ISBN 978-0-19-512579-5.
| Microbiology: Bacteria | |||||||
|---|---|---|---|---|---|---|---|
| Medical microbiology | |||||||
| Biochemistry and ecology |
| ||||||
| Shape | |||||||
| Structure |
| ||||||
| Taxonomy and evolution | |||||||