| |

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | O8S2 |
| Molar mass | 192.11 g·mol |
| Conjugate acid | Peroxydisulfuric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
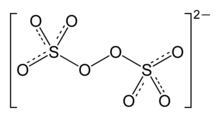
The peroxydisulfate ion, S
2O
8, is an oxyanion, the anion of peroxydisulfuric acid. It is commonly referred to as persulfate, but this term also refers to the peroxomonosulfate ion, SO
5. It is also called peroxodisulfate. Approximately 500,000 tons of salts containing this anion are produced annually. Important salts include sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), and ammonium persulfate ((NH4)2S2O8). These salts are colourless, water-soluble solids that are strong oxidants.
Applications
Salts of peroxydisulfate are mainly used to initiate the polymerization of various alkenes, including styrene, acrylonitrile, and fluoroalkenes. Polymerization is initiated by the homolysis of the peroxydisulfate:
- ⇌ 2
Moreover, sodium peroxydisulfate can be used for soil and groundwater remediation, water and wastewater treatment, and etching of copper on circuit boards.
It has also been used to produce hair lighteners and bleaches, medical drugs, cellophane, rubber, soaps, detergents, adhesive papers, dyes for textiles, and in photography.
In addition to its major commercial applications, peroxydisulfate participates in reactions of interest in the laboratory:
- Elbs persulfate oxidation
- Oxidation of Ag to Ag, such as the preparation of tetrakis(pyridine)silver(II) peroxydisulfate
Structure
Peroxydisulfate is a centrosymmetric anion. The O-O distance is 1.48 Å. The sulfur centers are tetrahedral.
References
- Ambiguous—see persulfate
- ^ Shafiee, Saiful Arifin; Aarons, Jolyon; Hamzah, Hairul Hisham (2018). "Electroreduction of Peroxodisulfate: A Review of a Complicated Reaction". Journal of the Electrochemical Society. 165 (13): H785–H798. doi:10.1149/2.1161811jes. S2CID 106396614.
- Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - Wacławek, Stanisław; Lutze, Holger V.; Grübel, Klaudiusz; Padil, Vinod V.T.; Černík, Miroslav; Dionysiou, Dionysios.D. (2017). "Chemistry of persulfates in water and wastewater treatment: A review". Chemical Engineering Journal. 330: 44–62. doi:10.1016/j.cej.2017.07.132.
- Allan, David R. (2006). "Sodium Peroxodisulfate". Acta Crystallographica Section E. 62 (3): i44–i46. doi:10.1107/S1600536806004302.
| Salts and covalent derivatives of the persulfate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |