| |
| Names | |
|---|---|
| IUPAC name tetraiodoplatinum | |
| Other names Platinum tetraiodide, platinic iodide, platinum(4+) tetraiodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.029.280 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | I4Pt |
| Molar mass | 702.702 g·mol |
| Appearance | brown crystals |
| Density | 6.06 g/cm |
| Melting point | 130 °C (266 °F; 403 K) |
| Solubility in water | decomposes in water |
| Related compounds | |
| Related compounds | Iridium tetraiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Platinum(IV) iodide is a inorganic compound with the formula PtI4. it is a dark brown diamagnetic solid and is one of several binary iodides of platinum.
Preparation
Platinum(IV) iodide can be prepared from the effect of iodine on platinum:
- Pt + 2I2 → PtI4
Iodide accelerates this process.
It can also be obtained from the decomposition of hydrogen hexaiodoplatinate(IV) at 80 °C:
- H2[PtI6] → PtI4 + 2HI
Physical properties
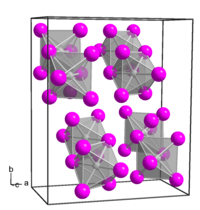
Platinum(IV) iodide forms dark brown crystals of several modifications:
- α-PtI4, rhombic crystal system, spatial group P bca, cell parameters a = 1.290 nm, b = 1.564 nm, c = 0.690 nm, Z = 8;
- β-PtI4, cubic crystal system, spatial group P m3m, cell parameters a = 0.56 nm, Z = 1;
- γ-PtI4, tetragonal crystal system, spatial group I 41/a, cell parameters a = 0.677 nm, c = 3.110 nm, Z = 8.
Platinum(IV) iodide decomposes in water. It is also soluble in ethanol, acetone, alkali, HI, KI, liquid NH3.
Chemical properties
It decomposes when heated:
- PtI4 → Pt + 2I2
When dissolved in hydroiodic acid, platinum(IV) iodide forms hydrogen hexaiodoplatinate(IV):
- PtI4 + 2HI → H2[PtI6]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Wicks, Charles E.; Block, Frank E. (1963). Thermodynamic Properties of 65 Elements: Their Oxides, Halides, Carbides and Nitrides. U.S. Government Printing Office. p. 92. Retrieved 28 March 2024.
- Olsen, Espen; Hagen, Georg; Eric Lindquist, Sten (2000). "Dissolution of platinum in methoxy propionitrile containing LiI/I2". Solar Energy Materials and Solar Cells. 63 (3): 267–273. doi:10.1016/s0927-0248(00)00033-7.
- Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 3510. ISBN 978-0-412-30120-9. Retrieved 28 March 2024.
- Donnay, Joseph Désiré Hubert (1978). Crystal Data: Inorganic compounds 1967-1969. National Bureau of Standards. p. 153. Retrieved 28 March 2024.
- "Platinum(IV) iodide, 99.95% (Metals basis), Pt 27.3% min., Thermo Scientific Chemicals, Premion | Fisher Scientific". Fisher Scientific. Retrieved 28 March 2024.
| Platinum compounds | |||
|---|---|---|---|
| Pt(−II) | |||
| Pt(0) | |||
| Pt(II) |
| ||
| Pt(IV) | |||
| Pt(V) | |||
| Pt(VI) | |||
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||