| |

| |
| Names | |
|---|---|
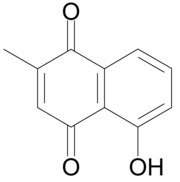
| Preferred IUPAC name 5-Hydroxy-2-methylnaphthalene-1,4-dione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.882 |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C11H8O3 |
| Molar mass | 188.17942 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Plumbagin or 5-hydroxy-2-methyl-1,4-naphthoquinone is an organic compound with the chemical formula C
11H
8O
3. It is regarded as a toxin and it is genotoxic and mutagenic.
Plumbagin is a yellow dye, formally derived from naphthoquinone.
It is named after the plant genus Plumbago, from which it was originally isolated. It is also commonly found in the carnivorous plant genera Drosera and Nepenthes. It is also a component of the black walnut drupe.
See also
References
- ^ Black Walnut. Drugs.com.
- Jemal Demma; Karl Hallberg; Björn Hellman (2009). "Genotoxicity of plumbagin and its effects on catechol and NQNO-induced DNA damage in mouse lymphoma cells". Toxicology in Vitro. 23 (2): 266–271. Bibcode:2009ToxVi..23..266D. doi:10.1016/j.tiv.2008.12.007. PMID 19124069.
- S B Farr; D O Natvig & T Kogoma (1985). "Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli". J Bacteriol. 164 (3): 1309–1316. doi:10.1128/JB.164.3.1309-1316.1985. PMC 219331. PMID 2933393.
- van der Vijver; L. M. (1972). "Distribution of Plumbagin in the Plumbaginaceae". Phytochemistry. 11 (11): 3247–3248. Bibcode:1972PChem..11.3247V. doi:10.1016/S0031-9422(00)86380-3.
- Wang, W.; Luo, X.; Li, H. (2010). "Terahertz and Infrared Spectra of Plumbagin, Juglone, and Menadione". Carnivorous Plant Newsletter. 39 (3): 82–88. doi:10.55360/cpn393.ww544.
- Rischer, H.; Hamm, A.; Bringmann, G. (2002). "Nepenthes insignis Uses a C2-Portion of the Carbon Skeleton of L-Alanine Acquired via its Carnivorous Organs, to Build up the Allelochemical Plumbagin". Phytochemistry. 59 (6): 603–609. Bibcode:2002PChem..59..603R. doi:10.1016/S0031-9422(02)00003-1. PMID 11867092.