| |
| Names | |
|---|---|
| Other names Polyethylene furanoate; Polyethylene furandicarboxylate; Poly(ethylene furanoate) | |
| Identifiers | |
| CAS Number | |
| Properties | |
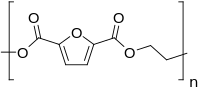
| Chemical formula | (C8H6O5)n |
| Molar mass | Variable |
| Density | 1.43 g/cm |
| Melting point | 195–265 °C (383–509 °F; 468–538 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Polyethylene furan-2,5-dicarboxylate, also named poly(ethylene furan-2,5-dicarboxylate), polyethylene furanoate and poly(ethylene furanoate) and generally abbreviated as PEF, is a polymer that can be produced by polycondensation or ring-opening polymerization of 2,5-furandicarboxylic acid (FDCA) and ethylene glycol. As an aromatic polyester from ethylene glycol it is a chemical analogue of polyethylene terephthalate (PET) and polyethylene naphthalate (PEN). PEF has been described in (patent) literature since 1951, but has gained renewed attention since the US department of energy proclaimed its building block, FDCA, as a potential bio-based replacement for purified terephthalic acid (PTA) in 2004.
Benefits over PET
One life-cycle assessment showed that replacing PTA in the production of PET by bio-based FDCA for the production of PEF has a potential for significant reductions in greenhouse gas (GHG) emissions and non-renewable energy use (NREU). Furthermore, PEF exhibits an intrinsically higher gas barrier for oxygen, carbon dioxide and water vapor than PET and is therefore an interesting alternative for packaging applications such as bottles, films and food trays.
References
- ^ "PEF". Avalon Industries.
- ^ de Jong, E.; Dam, M. A.; Sipos, L.; Gruter, G.-J. M. (January 2012). "Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters". ACS Symposium Series. 1105: 1–13. doi:10.1021/BK-2012-1105.CH001.
- J.-G. Rosenboom et al., Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers, Nature Communications, 2018
- US 2551731 A, Polyesters from heterocyclic components, 1951
- Top Value Added Chemicals from Biomass
- A.J.J.E. Eerhart et al., Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance, Energy Environ. Sci., 2012
- S.K. Burgess et al., Oxygen sorption and transport in amorphous poly (ethylene furanoate), Polymer, 2014
- S.K. Burgess et al., Carbon Dioxide Sorption and Transport in Amorphous Poly (ethylene furanoate), Macromolecules, 2015
- S.K. Burgess et al., Water sorption in poly (ethylene furanoate) compared to poly (ethylene terephthalate). Part 2: Kinetic sorption, Polymer, 2014