"SSRI" redirects here. For other uses, see SSRI (disambiguation).
| Selective serotonin reuptake inhibitor | |
|---|---|
| Drug class | |
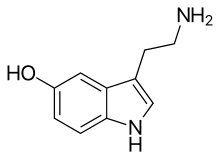 Serotonin, the neurotransmitter that is involved in the mechanism of action of SSRIs Serotonin, the neurotransmitter that is involved in the mechanism of action of SSRIs | |
| Class identifiers | |
| Synonyms | Serotonin-specific reuptake inhibitors, serotonergic antidepressants |
| Use | Major depressive disorder, anxiety disorders |
| ATC code | N06AB |
| Biological target | Serotonin transporter |
| Clinical data | |
| Drugs.com | Drug Classes |
| Consumer Reports | Best Buy Drugs |
| External links | |
| MeSH | D017367 |
| Legal status | |
| In Wikidata | |
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
SSRIs increase the extracellular level of the neurotransmitter serotonin by limiting its reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine and dopamine transporters.
SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations.
Medical uses
The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for anxiety disorders, such as social anxiety disorder, generalized anxiety disorder, panic disorder, obsessive–compulsive disorder (OCD), eating disorders, chronic pain, and, in some cases, for posttraumatic stress disorder (PTSD). They are also frequently used to treat depersonalization disorder, although with varying results.
Depression
Antidepressants are recommended by the UK National Institute for Health and Care Excellence (NICE) as a first-line treatment of severe depression and for the treatment of mild-to-moderate depression that persists after conservative measures such as cognitive therapy. They recommend against their routine use by those who have chronic health problems and mild depression.
There has been controversy regarding the efficacy of SSRIs in treating depression depending on its severity and duration.
- Two meta-analyses published in 2008 (Kirsch) and 2010 (Fournier) found that in mild and moderate depression, the effect of SSRIs is small or none compared to placebo, while in very severe depression the effect of SSRIs is between "relatively small" and "substantial". The 2008 meta-analysis combined 35 clinical trials submitted to the Food and Drug Administration (FDA) before licensing of four newer antidepressants (including the SSRIs paroxetine and fluoxetine, the non-SSRI antidepressant nefazodone, and the serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine). The authors attributed the relationship between severity and efficacy to a reduction of the placebo effect in severely depressed patients, rather than an increase in the effect of the medication. Some researchers have questioned the statistical basis of this study suggesting that it underestimates the effect size of antidepressants.
- A 2012 meta-analysis of fluoxetine and venlafaxine concluded that statistically and clinically significant treatment effects were observed for each drug relative to placebo irrespective of baseline depression severity; some of the authors however disclosed substantial relationships with pharmaceutical industries.
- A 2017 systematic review stated that "SSRIs versus placebo seem to have statistically significant effects on depressive symptoms, but the clinical significance of these effects seems questionable and all trials were at high risk of bias. Furthermore, SSRIs versus placebo significantly increase the risk of both serious and non-serious adverse events. Our results show that the harmful effects of SSRIs versus placebo for major depressive disorder seem to outweigh any potentially small beneficial effects". Fredrik Hieronymus et al. criticized the review as inaccurate and misleading, but they also disclosed multiple ties to pharmaceutical industries and receipt of speaker's fees.
- In 2018, a systematic review and network meta-analysis comparing the efficacy and acceptability of 21 antidepressant drugs showed escitalopram to be one of the most effective. They showed that "In terms of efficacy, all antidepressants were more effective than placebo, with odds ratios (ORs) ranging between 2.13 (95% credible interval 1.89–2.41) for amitriptyline and 1.37 (1.16–1.63) for reboxetine." The odds ratios were specifically in terms of response rates (≥50% reduction in observer-rated symptoms). Odds ratios of response rates have been criticized for artificially inflating the apparent size of antidepressant benefits.
The use of SSRIs in children with depression remains controversial. A 2021 Cochrane review concluded that, for children and adolescents, SSRIs "may reduce depression symptoms in a small and unimportant way compared with placebo." However, it also noted significant methodological limitations that make drawing definitive conclusions about efficacy difficult. Fluoxetine is the only SSRI authorized for use in children and adolescents with moderate to severe depression in the United Kingdom.
Social anxiety disorder
Some SSRIs are effective for social anxiety disorder, although their effects on symptoms is not always robust and their use is sometimes rejected in favor of psychological therapies. Paroxetine was the first drug to be approved for social anxiety disorder and it is considered effective for this disorder; sertraline and fluvoxamine were later approved for it as well. Escitalopram and citalopram are used off-label with acceptable efficacy, while fluoxetine is not considered to be effective for this disorder. The effect sizes (Cohen's d) of SSRIs in terms of improvement on the Liebowitz social anxiety scale in individual published trials of the drugs for social anxiety disorder have ranged from –0.029 to 1.214.
Post-traumatic stress disorder
PTSD is relatively hard to treat and generally treatment is not highly effective; SSRIs are no exception. They are not very effective for this disorder and only two SSRIs are FDA approved for this condition: paroxetine and sertraline. Paroxetine has slightly higher response and remission rates for PTSD than sertraline, but both are not fully effective for many patients. Fluoxetine is used off-label, but with mixed results; venlafaxine, an SNRI, is considered somewhat effective, although its use is also off-label. Fluvoxamine, escitalopram and citalopram are not well tested in this disorder. Paroxetine remains the most suitable drug for PTSD as of now, but with limited benefits.
Generalized anxiety disorder
SSRIs are recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of generalized anxiety disorder (GAD) that has failed to respond to conservative measures such as education and self-help activities. GAD is a common disorder of which the central feature is excessive worry about a number of different events. Key symptoms include excessive anxiety about multiple events and issues, and difficulty controlling worrisome thoughts, that persists for at least 6 months.
Antidepressants provide a modest-to-moderate reduction in anxiety in GAD, and are superior to placebo in treating GAD. The efficacy of different antidepressants is similar.
Obsessive–compulsive disorder
In Canada, SSRIs are a first-line treatment of adult obsessive–compulsive disorder (OCD). In the UK, they are first-line treatment only with moderate to severe functional impairment and as second line treatment for those with mild impairment, though, as of early 2019, this recommendation is being reviewed. In children, SSRIs can be considered a second line therapy in those with moderate-to-severe impairment, with close monitoring for psychiatric adverse effects. SSRIs, especially fluvoxamine, which is the first one to be FDA approved for OCD, are efficacious in its treatment; patients treated with SSRIs are about twice as likely to respond to treatment as those treated with placebo. Efficacy has been demonstrated both in short-term treatment trials of 6 to 24 weeks and in discontinuation trials of 28 to 52 weeks duration.
Panic disorder
Paroxetine CR was superior to placebo on the primary outcome measure. In a 10-week randomized controlled, double-blind trial escitalopram was more effective than placebo. Fluvoxamine, another SSRI, has shown positive results. However, evidence for their effectiveness and acceptability is unclear.
Eating disorders
Antidepressants are recommended as an alternative or additional first step to self-help programs in the treatment of bulimia nervosa. SSRIs (fluoxetine in particular) are preferred over other anti-depressants due to their acceptability, tolerability, and superior reduction of symptoms in short-term trials. Long-term efficacy remains poorly characterized.
Similar recommendations apply to binge eating disorder. SSRIs provide short-term reductions in binge eating behavior, but have not been associated with significant weight loss.
Clinical trials have generated mostly negative results for the use of SSRIs in the treatment of anorexia nervosa. Treatment guidelines from the National Institute of Health and Clinical Excellence recommend against the use of SSRIs in this disorder. Those from the American Psychiatric Association note that SSRIs confer no advantage regarding weight gain, but that they may be used for the treatment of co-existing depression, anxiety, or OCD.
Stroke recovery
SSRIs have been used off-label in the treatment of stroke patients, including those with and without symptoms of depression. A 2021 meta-analysis of randomized controlled clinical trials found no evidence pointing to their routine use to promote recovery following stroke.
Premature ejaculation
Main article: Premature ejaculationSSRIs are effective for the treatment of premature ejaculation. Taking SSRIs on a chronic, daily basis is more effective than taking them prior to sexual activity. The increased efficacy of treatment when taking SSRIs on a daily basis is consistent with clinical observations that the therapeutic effects of SSRIs generally take several weeks to emerge. Sexual dysfunction ranging from decreased libido to anorgasmia is usually considered to be a significantly distressing side effect which may lead to noncompliance in patients receiving SSRIs. However, for those with premature ejaculation, this very same side effect becomes the desired effect.
Other uses
SSRIs such as sertraline have been found to be effective in decreasing anger.
Side effects
Side effects vary among the individual drugs of this class. They may include akathisia.
Sexual dysfunction
SSRIs can cause various types of sexual dysfunction such as anorgasmia, erectile dysfunction, diminished libido, genital numbness, and sexual anhedonia (pleasureless orgasm). Sexual problems are common with SSRIs. Poor sexual function is one of the most common reasons people stop the medication.
The mechanism by which SSRIs may cause sexual side effects is not well understood as of 2021. The range of possible mechanisms includes (1) nonspecific neurological effects (e.g., sedation) that globally impair behavior including sexual function; (2) specific effects on brain systems mediating sexual function; (3) specific effects on peripheral tissues and organs, such as the penis, that mediate sexual function; and (4) direct or indirect effects on hormones mediating sexual function. Management strategies include: for erectile dysfunction the addition of a PDE5 inhibitor such as sildenafil; for decreased libido, possibly adding or switching to bupropion; and for overall sexual dysfunction, switching to nefazodone. Buspirone is sometimes used off-label to reduce sexual dysfunction associated with the use of SSRIs.
A number of non-SSRI drugs are not associated with sexual side effects (such as bupropion, mirtazapine, tianeptine, agomelatine, tranylcypromine, and moclobemide).
Several studies have suggested that SSRIs may adversely affect semen quality.
While trazodone (an antidepressant with alpha adrenergic receptor blockade) is a notorious cause of priapism, cases of priapism have also been reported with certain SSRIs (e.g. fluoxetine, citalopram).
Post-SSRI sexual dysfunction
Post-SSRI sexual dysfunction (PSSD) refers to a set of symptoms reported by some people who have taken SSRIs or other serotonin reuptake-inhibiting (SRI) drugs, in which sexual dysfunction symptoms persist for at least three months after ceasing to take the drug. The status of PSSD as a legitimate and distinct pathology is contentious; several researchers have proposed that it should be recognized as a separate phenomenon from more common SSRI side effects.
The reported symptoms of PSSD include reduced sexual desire or arousal, erectile dysfunction in males or loss of vaginal lubrication in females, difficulty having an orgasm or loss of pleasurable sensation associated with orgasm, and a reduction or loss of sensitivity in the genitals or other erogenous zones. Additional non-sexual symptoms are also commonly described, including emotional numbing, anhedonia, depersonalization or derealization, and cognitive impairment. The duration of PSSD symptoms appears to vary among patients, with some cases resolving in months and others in years or decades; one analysis of patient reports submitted between 1992 and 2021 in the Netherlands listed a case which had reportedly persisted for 23 years. The symptoms of PSSD are largely shared with post-finasteride syndrome (PFS) and post-retinoid sexual dysfunction (PRSD), two other poorly-understood conditions which have been suggested to share a common etiology with PSSD despite being associated with different types of medication.
Diagnostic criteria for PSSD were proposed in 2022, but as of 2023, there is no agreement on standards for diagnosis. It is considered a distinct phenomenon from antidepressant discontinuation syndrome, post-acute withdrawal syndrome, and major depressive disorder, and should be distinguished from sexual dysfunction associated with depression and persistent genital arousal disorder. There are limited treatment options for PSSD as of 2023 and no evidence that any individual approach is effective. The mechanism by which SRIs may induce PSSD is unclear; neurobiological and cognitive factors may act in combination to cause the problem. As of 2023, prevalence is unknown. A 2020 review stated that PSSD is rare, underreported, and "increasingly identified in online communities". A 2024 study investigating the prevalence of persistent post-treatment genital numbness among sexual and gender minority youth found 13.2% of SSRI users between the ages 15 and 29 reporting the symptom compared to 0.9% who had used other medications.
Reports of PSSD have occurred with almost every SSRI (dapoxetine is an exception). In 2019, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency (EMA) recommended that packaging leaflets of selected SSRIs and SNRIs should be amended to include information regarding a possible risk of persistent sexual dysfunction. Following the EMA assessment, a safety review by Health Canada "could neither confirm nor rule out a causal link ... which was long lasting in rare cases", but recommended that "healthcare professionals inform patients about the potential risk of long-lasting sexual dysfunction despite discontinuation of treatment". A 2023 review stated that ongoing sexual dysfunction after SSRI discontinuation was possible, but that cause and effect were undetermined. The 2023 review cautioned that reports of sexual dysfunction cannot be generalized to wider practice as they are subject to a "high risk of bias", but agreed with the EMA assessment that cautionary labeling on SSRIs was warranted.
On the 20th of march of 2024, a lawsuit was filed by the organization Public Citizen, representing Dr. Antonei Csoka, against the United States Food and Drug Administration (FDA) for failing to act on a citizen petition submitted in 2018. The petition seeks to have the risk of serious sexual side effects persisting after discontinuation mentioned in the product labels of SSRIs and SNRIs.
Emotional blunting
Certain antidepressants may cause emotional blunting, characterized by reduced intensity of both positive and negative emotions as well as symptoms of apathy, indifference, and amotivation. It may be experienced as either beneficial or detrimental depending on the situation. This side effect has been particularly associated with serotonergic antidepressants like SSRIs and SNRIs, but may be less with atypical antidepressants like bupropion, agomelatine, and vortioxetine. Higher doses of antidepressants seem to be more likely to produce emotional blunting than lower doses. It can be decreased by reducing dosage, discontinuing the medication, or switching to a different antidepressant that may have less propensity for causing this side effect.
Vision
Acute narrow-angle glaucoma is the most common and important ocular side effect of SSRIs, and often goes misdiagnosed.
Cardiac
SSRIs do not appear to affect the risk of coronary heart disease (CHD) in those without a previous diagnosis of CHD. A large cohort study suggested no substantial increase in the risk of cardiac malformations attributable to SSRI usage during the first trimester of pregnancy. A number of large studies of people without known pre-existing heart disease have reported no EKG changes related to SSRI use. The recommended maximum daily dose of citalopram and escitalopram was reduced due to concerns with QT prolongation. In overdose, fluoxetine has been reported to cause sinus tachycardia, myocardial infarction, junctional rhythms, and trigeminy. Some authors have suggested electrocardiographic monitoring in patients with severe pre-existing cardiovascular disease who are taking SSRIs.
In a 2023 study a possible connection between SSRI usage and the onset of mitral valve regurgitation was identified, indicating that SSRIs could hasten the progression of degenerative mitral valve regurgitation (DMR), especially in individuals carrying 5-HTTLPR genotype. The study’s authors suggest that genotyping should be performed on people with DMR to evaluate serotonin transporter (SERT) activity. They also urge practitioners to exercise caution when prescribing SSRIs to individuals with a familial history of DMR.
Bleeding
SSRIs directly increase the risk of abnormal bleeding by lowering platelet serotonin levels, which are essential to platelet-driven hemostasis. SSRIs interact with anticoagulants, like warfarin, and antiplatelet drugs, like aspirin. This includes an increased risk of GI bleeding, and post operative bleeding. The relative risk of intracranial bleeding is increased, but the absolute risk is very low. SSRIs are known to cause platelet dysfunction. This risk is greater in those who are also on anticoagulants, antiplatelet agents and NSAIDs (nonsteroidal anti-inflammatory drugs), as well as with the co-existence of underlying diseases such as cirrhosis of the liver or liver failure.
Fracture risk
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests an association between SSRI usage at therapeutic doses and a decrease in bone mineral density, as well as increased fracture risk, a relationship that appears to persist even with adjuvant bisphosphonate therapy. However, because the relationship between SSRIs and fractures is based on observational data as opposed to prospective trials, the phenomenon is not definitively causal. There also appears to be an increase in fracture-inducing falls with SSRI use, suggesting the need for increased attention to fall risk in elderly patients using the medication. The loss of bone density does not appear to occur in younger patients taking SSRIs.
Bruxism
SSRI and SNRI antidepressants may cause jaw pain/jaw spasm reversible syndrome (although it is not common). Buspirone appears to be successful in treating bruxism on SSRI/SNRI induced jaw clenching.
Serotonin syndrome
Main article: Serotonin syndromeSerotonin syndrome is typically caused by the use of two or more serotonergic drugs, including SSRIs. Serotonin syndrome is a condition that can range from mild (most common) to deadly. Mild symptoms may consist of increased heart rate, fever, shivering, sweating, dilated pupils, myoclonus (intermittent jerking or twitching), as well as hyperreflexia. Concomitant use of SSRIs or SNRIs for depression with a triptan for migraine does not appear to heighten the risk of the serotonin syndrome. Taking monoamine oxidase inhibitors (MAOIs) in combination with SSRIs can be fatal, since MAOIs disrupt monoamine oxidase, an enzyme which is needed to break down serotonin and other neurotransmitters. Without monoamine oxidase, the body is unable to eliminate excess neurotransmitters, allowing them to build up to dangerous levels. The prognosis for recovery in a hospital setting is generally good if serotonin syndrome is correctly identified. Treatment consists of discontinuing any serotonergic drugs and providing supportive care to manage agitation and hyperthermia, usually with benzodiazepines.
Suicide risk
Children and adolescents
Meta analyses of short duration randomized clinical trials have found that SSRI use is related to a higher risk of suicidal behavior in children and adolescents. For instance, a 2004 U.S. Food and Drug Administration (FDA) analysis of clinical trials on children with major depressive disorder found statistically significant increases of the risks of "possible suicidal ideation and suicidal behavior" by about 80%, and of agitation and hostility by about 130%. According to the FDA, the heightened risk of suicidality is within the first one to two months of treatment. The National Institute for Health and Care Excellence (NICE) places the excess risk in the "early stages of treatment". The European Psychiatric Association places the excess risk in the first two weeks of treatment and, based on a combination of epidemiological, prospective cohort, medical claims, and randomized clinical trial data, concludes that a protective effect dominates after this early period. A 2014 Cochrane review found that at six to nine months, suicidal ideation remained higher in children treated with antidepressants compared to those treated with psychological therapy.
A recent comparison of aggression and hostility occurring during treatment with fluoxetine to placebo in children and adolescents found that no significant difference between the fluoxetine group and a placebo group. There is also evidence that higher rates of SSRI prescriptions are associated with lower rates of suicide in children, though since the evidence is correlational, the true nature of the relationship is unclear.
In 2004, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom judged fluoxetine (Prozac) to be the only antidepressant that offered a favorable risk-benefit ratio in children with depression, though it was also associated with a slight increase in the risk of self-harm and suicidal ideation. Only two SSRIs are licensed for use with children in the UK, sertraline (Zoloft) and fluvoxamine (Luvox), for the treatment of obsessive–compulsive disorder. Fluoxetine is not licensed for this use.
Adults
It is unclear whether SSRIs affect the risk of suicidal behavior in adults.
- A 2005 meta-analysis of drug company data found no evidence that SSRIs increased the risk of suicide; however, important protective or hazardous effects could not be excluded.
- A 2005 review observed that suicide attempts are increased in those who use SSRIs as compared to placebo and compared to therapeutic interventions other than tricyclic antidepressants. No difference risk of suicide attempts was detected between SSRIs versus tricyclic antidepressants.
- A 2006 review suggests that the widespread use of antidepressants in the new "SSRI-era" appears to have led to a highly significant decline in suicide rates in most countries with traditionally high baseline suicide rates. The decline is particularly striking for women who, compared with men, seek more help for depression. Recent clinical data on large samples in the US too have revealed a protective effect of antidepressant against suicide.
- A 2006 meta-analysis of randomized controlled trials suggests that SSRIs increase suicide ideation compared with placebo. However, the observational studies suggest that SSRIs did not increase suicide risk more than older antidepressants. The researchers stated that if SSRIs increase suicide risk in some patients, the number of additional deaths is very small because ecological studies have generally found that suicide mortality has declined (or at least not increased) as SSRI use has increased.
- An additional meta-analysis by the FDA in 2006 found an age-related effect of SSRI's. Among adults younger than 25 years, results indicated that there was a higher risk for suicidal behavior. For adults between 25 and 64, the effect appears neutral on suicidal behavior but possibly protective for suicidal behavior for adults between the ages of 25 and 64. For adults older than 64, SSRI's seem to reduce the risk of both suicidal behavior.
- In 2016 a study criticized the effects of the FDA Black Box suicide warning inclusion in the prescription. The authors discussed the suicide rates might increase also as a consequence of the warning.
Risk of death
A 2017 meta-analysis found that antidepressants including SSRIs were associated with significantly increased risk of death (+33%) and new cardiovascular complications (+14%) in the general population. Conversely, risks were not greater in people with existing cardiovascular disease.
Pregnancy and breastfeeding
SSRI use in pregnancy has been associated with a variety of risks with varying degrees of proof of causation. As depression is independently associated with negative pregnancy outcomes, determining the extent to which observed associations between antidepressant use and specific adverse outcomes reflects a causative relationship has been difficult in some cases. In other cases, the attribution of adverse outcomes to antidepressant exposure seems fairly clear.
SSRI use in pregnancy is associated with an increased risk of spontaneous abortion of about 1.7-fold. Use is also associated with preterm birth. According to some researches, decreased body weight of the child, intrauterine growth retardation, neonatal adaptive syndrome, and persistent pulmonary hypertension also was noted.
A systematic review of the risk of major birth defects in antidepressant-exposed pregnancies found a small increase (3% to 24%) in the risk of major malformations and a risk of cardiovascular birth defects that did not differ from non-exposed pregnancies. Other studies have found an increased risk of cardiovascular birth defects among depressed mothers not undergoing SSRI treatment, suggesting the possibility of ascertainment bias, e.g. that worried mothers may pursue more aggressive testing of their infants. Another study found no increase in cardiovascular birth defects and a 27% increased risk of major malformations in SSRI exposed pregnancies.
The FDA issued a statement on July 19, 2006, stating nursing mothers on SSRIs must discuss treatment with their physicians. However, the medical literature on the safety of SSRIs has determined that some SSRIs like Sertraline and Paroxetine are considered safe for breastfeeding.
Neonatal abstinence syndrome
Several studies have documented neonatal abstinence syndrome, a syndrome of neurological, gastrointestinal, autonomic, endocrine and/or respiratory symptoms among a large minority of infants with intrauterine exposure. These syndromes are short-lived, but insufficient long-term data is available to determine whether there are long-term effects.
Persistent pulmonary hypertension
Persistent pulmonary hypertension (PPHN) is a serious and life-threatening, but very rare, lung condition that occurs soon after birth of the newborn. Newborn babies with PPHN have high pressure in their lung blood vessels and are not able to get enough oxygen into their bloodstream. About 1 to 2 babies per 1000 babies born in the U.S. develop PPHN shortly after birth, and often they need intensive medical care. It is associated with about a 25% risk of significant long-term neurological deficits. A 2014 meta analysis found no increased risk of persistent pulmonary hypertension associated with exposure to SSRI's in early pregnancy and a slight increase in risk associates with exposure late in pregnancy; "an estimated 286 to 351 women would need to be treated with an SSRI in late pregnancy to result in an average of one additional case of persistent pulmonary hypertension of the newborn". A review published in 2012 reached conclusions very similar to those of the 2014 study.
Neuropsychiatric effects in offspring
According to a 2015 review available data found that "some signal exists suggesting that antenatal exposure to SSRIs may increase the risk of ASDs (autism spectrum disorders)" even though a large cohort study published in 2013 and a cohort study using data from Finland's national register between the years 1996 and 2010 and published in 2016 found no significant association between SSRI use and autism in offspring. The 2016 Finland study also found no association with ADHD, but did find an association with increased rates of depression diagnoses in early adolescence.
Bipolar switch
In adults and children with bipolar disorder, SSRIs may cause a bipolar switch from depression into hypomania/mania, mixed states or rapid cycling. When taken with mood stabilizers, the risk of switching is not increased, however when taking SSRIs as a monotherapy, the risk of switching may be twice or three times that of the average. The changes are not often easy to detect and require monitoring by family and mental health professionals. This switch might happen even with no prior (hypo)manic episodes and might therefore not be foreseen by the psychiatrist.
Interactions
The following drugs may precipitate serotonin syndrome in people on SSRIs:
- Linezolid
- Monoamine oxidase inhibitors (MAOIs) including moclobemide, phenelzine, tranylcypromine, selegiline and methylene blue
- Lithium
- Sibutramine
- MDMA (ecstasy)
- Dextromethorphan
- Tramadol
- 5-HTP
- Pethidine/meperidine
- St. John's wort
- Yohimbe
- Tricyclic antidepressants (TCAs)
- Serotonin-norepinephrine reuptake inhibitors (SNRIs)
- Buspirone
- Triptan
- Mirtazapine
- Methylene blue
Painkillers of the NSAIDs drug family may interfere and reduce efficiency of SSRIs and may compound the increased risk of gastrointestinal bleeds caused by SSRI use. NSAIDs include:
There are a number of potential pharmacokinetic interactions between the various individual SSRIs and other medications. Most of these arise from the fact that every SSRI has the ability to inhibit certain P450 cytochrome enzymes.
| Drug name | CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4 | CYP2B6 |
|---|---|---|---|---|---|---|
| Citalopram | + | 0 | 0 | + | 0 | 0 |
| Escitalopram | 0 | 0 | 0 | + | 0 | 0 |
| Fluoxetine | + | ++ | +/++ | +++ | + | + |
| Fluvoxamine | +++ | ++ | +++ | + | + | + |
| Paroxetine | + | + | + | +++ | + | +++ |
| Sertraline | + | + | +/++ | + | + | + |
Legend:
0 – no inhibition
+ – mild/weak inhibition
++ – moderate inhibition
+++ – strong/potent inhibition
The CYP2D6 enzyme is entirely responsible for the metabolism of hydrocodone, codeine and dihydrocodeine to their active metabolites (hydromorphone, morphine, and dihydromorphine, respectively), which in turn undergo phase 2 glucuronidation. These opioids (and to a lesser extent oxycodone, tramadol, and methadone) have interaction potential with selective serotonin reuptake inhibitors. The concomitant use of some SSRIs (paroxetine and fluoxetine) with codeine may decrease the plasma concentration of active metabolite morphine, which may result in reduced analgesic efficacy.
Another important interaction of certain SSRIs involves paroxetine, a potent inhibitor of CYP2D6, and tamoxifen, an agent used commonly in the treatment and prevention of breast cancer. Tamoxifen is a prodrug that is metabolised by the hepatic cytochrome P450 enzyme system, especially CYP2D6, to its active metabolites. Concomitant use of paroxetine and tamoxifen in women with breast cancer is associated with a higher risk of death, as much as a 91 percent in women who used it the longest.
Overdose
See also: Serotonin syndromeSSRIs appear safer in overdose when compared with traditional antidepressants, such as the tricyclic antidepressants. This relative safety is supported both by case series and studies of deaths per numbers of prescriptions. However, case reports of SSRI poisoning have indicated that severe toxicity can occur and deaths have been reported following massive single ingestions, although this is exceedingly uncommon when compared to the tricyclic antidepressants.
Because of the wide therapeutic index of the SSRIs, most patients will have mild or no symptoms following moderate overdoses. The most commonly reported severe effect following SSRI overdose is serotonin syndrome; serotonin toxicity is usually associated with very high overdoses or multiple drug ingestion. Other reported significant effects include coma, seizures, and cardiac toxicity.
Poisoning is also known in animals, and some toxicity information is available for veterinary treatment.
Discontinuation syndrome
Main article: SSRI discontinuation syndromeSerotonin reuptake inhibitors should not be abruptly discontinued after extended therapy, and whenever possible, should be tapered over several weeks to minimize discontinuation-related symptoms which may include nausea, headache, dizziness, chills, body aches, paresthesias, insomnia, and brain zaps. Paroxetine may produce discontinuation-related symptoms at a greater rate than other SSRIs, though qualitatively similar effects have been reported for all SSRIs. Discontinuation effects appear to be less for fluoxetine, perhaps owing to its long half-life and the natural tapering effect associated with its slow clearance from the body. One strategy for minimizing SSRI discontinuation symptoms is to switch the patient to fluoxetine and then taper and discontinue the fluoxetine.
Mechanism of action
See also: Pharmacology of antidepressantsSerotonin reuptake inhibition
In the brain, messages are passed from a nerve cell to another via a chemical synapse, a small gap between the cells. The presynaptic cell that sends the information releases neurotransmitters including serotonin into that gap. The neurotransmitters are then recognized by receptors on the surface of the recipient postsynaptic cell, which upon this stimulation, in turn, relays the signal. About 10% of the neurotransmitters are lost in this process; the other 90% are released from the receptors and taken up again by monoamine transporters into the sending presynaptic cell, a process called reuptake.
SSRIs inhibit the reuptake of serotonin. As a result, the serotonin stays in the synaptic gap longer than it normally would, and may repeatedly stimulate the receptors of the recipient cell. In the short run, this leads to an increase in signaling across synapses in which serotonin serves as the primary neurotransmitter. On chronic dosing, the increased occupancy of post-synaptic serotonin receptors signals the pre-synaptic neuron to synthesize and release less serotonin. Serotonin levels within the synapse drop, then rise again, ultimately leading to downregulation of post-synaptic serotonin receptors. Other, indirect effects may include increased norepinephrine output, increased neuronal cyclic AMP levels, and increased levels of regulatory factors such as BDNF and CREB. Owing to the lack of a widely accepted comprehensive theory of the biology of mood disorders, there is no widely accepted theory of how these changes lead to the mood-elevating and anti-anxiety effects of SSRIs.
Their effects on serotonin blood levels, which take weeks to take effect, appear to be largely responsible for their slow-to-appear psychiatric effects. SSRIs mediate their action largely with high occupancy in a total of all serotonin transporters within the brain and through this slow downstream changes of large brain regions at therapeutic concentrations, whereas MDMA leads to an excess serotonin release in a short run. This could explain the absence of a "high" by antidepressants and in addition the contrary ability of SSRIs in expressing neuroprotective actions to the neurotoxic abilities of MDMA.
Sigma receptor ligands
| Medication | SERTTooltip Serotonin transporter | σ1 | σ2 | σ1 / SERT | |
|---|---|---|---|---|---|
| Citalopram | 1.16 | 292–404 | Agonist | 5,410 | 252–348 |
| Escitalopram | 2.5 | 288 | Agonist | ND | ND |
| Fluoxetine | 0.81 | 191–240 | Agonist | 16,100 | 296–365 |
| Fluvoxamine | 2.2 | 17–36 | Agonist | 8,439 | 7.7–16.4 |
| Paroxetine | 0.13 | ≥1,893 | ND | 22,870 | ≥14,562 |
| Sertraline | 0.29 | 32–57 | Antagonist | 5,297 | 110–197 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||
In addition to their actions as reuptake inhibitors of serotonin, some SSRIs are also, coincidentally, ligands of the sigma receptors. Fluvoxamine is an agonist of the σ1 receptor, while sertraline is an antagonist of the σ1 receptor, and paroxetine does not significantly interact with the σ1 receptor. None of the SSRIs have significant affinity for the σ2 receptor. Fluvoxamine has by far the strongest activity of the SSRIs at the σ1 receptor. High occupancy of the σ1 receptor by clinical dosages of fluvoxamine has been observed in the human brain in positron emission tomography (PET) research. It is thought that agonism of the σ1 receptor by fluvoxamine may have beneficial effects on cognition. In contrast to fluvoxamine, the relevance of the σ1 receptor in the actions of the other SSRIs is uncertain and questionable due to their very low affinity for the receptor relative to the SERT.
Anti-inflammatory effects
The role of inflammation and the immune system in depression has been extensively studied. The evidence supporting this link has been shown in numerous studies over the past ten years. Nationwide studies and meta-analyses of smaller cohort studies have uncovered a correlation between pre-existing inflammatory conditions such as type 1 diabetes, rheumatoid arthritis (RA), or hepatitis, and an increased risk of depression. Data also shows that using pro-inflammatory agents in the treatment of diseases like melanoma can lead to depression. Several meta-analytical studies have found increased levels of proinflammatory cytokines and chemokines in depressed patients. This link has led scientists to investigate the effects of antidepressants on the immune system.
SSRIs were originally invented with the goal of increasing levels of available serotonin in the extracellular spaces. However, the delayed response between when patients first begin SSRI treatment to when they see effects has led scientists to believe that other molecules are involved in the efficacy of these drugs. To investigate the apparent anti-inflammatory effects of SSRIs, both Kohler et al. and Więdłocha et al. conducted meta-analyses which have shown that after antidepressant treatment the levels of cytokines associated with inflammation are decreased. A large cohort study conducted by researchers in the Netherlands investigated the association between depressive disorders, symptoms, and antidepressants with inflammation. The study showed decreased levels of interleukin (IL)-6, a cytokine that has proinflammatory effects, in patients taking SSRIs compared to non-medicated patients.
Treatment with SSRIs has shown reduced production of inflammatory cytokines such as IL-1β, tumor necrosis factor (TNF)-α, IL-6, and interferon (IFN)-γ, which leads to a decrease in inflammation levels and subsequently a decrease in the activation level of the immune response. These inflammatory cytokines have been shown to activate microglia which are specialized macrophages that reside in the brain. Macrophages are a subset of immune cells responsible for host defense in the innate immune system. Macrophages can release cytokines and other chemicals to cause an inflammatory response. Peripheral inflammation can induce an inflammatory response in microglia and can cause neuroinflammation. SSRIs inhibit proinflammatory cytokine production which leads to less activation of microglia and peripheral macrophages. SSRIs not only inhibit the production of these proinflammatory cytokines, they also have been shown to upregulate anti-inflammatory cytokines such as IL-10. Taken together, this reduces the overall inflammatory immune response.
In addition to affecting cytokine production, there is evidence that treatment with SSRIs has effects on the proliferation and viability of immune system cells involved in both innate and adaptive immunity. Evidence shows that SSRIs can inhibit proliferation in T-cells, which are important cells for adaptive immunity and can induce inflammation. SSRIs can also induce apoptosis, programmed cell death, in T-cells. The full mechanism of action for the anti-inflammatory effects of SSRIs is not fully known. However, there is evidence for various pathways to have a hand in the mechanism. One such possible mechanism is the increased levels of cyclic adenosine monophosphate (cAMP) as a result of interference with activation of protein kinase A (PKA), a cAMP dependent protein. Other possible pathways include interference with calcium ion channels, or inducing cell death pathways like MAPK and Notch signaling pathway.
The anti-inflammatory effects of SSRIs have prompted studies of the efficacy of SSRIs in the treatment of autoimmune diseases such as multiple sclerosis, RA, inflammatory bowel diseases, and septic shock. These studies have been performed in animal models but have shown consistent immune regulatory effects. Fluoxetine, an SSRI, has also shown efficacy in animal models of graft vs. host disease. SSRIs have also been used successfully as pain relievers in patients undergoing oncology treatment. The effectiveness of this has been hypothesized to be at least in part due to the anti-inflammatory effects of SSRIs.
Pharmacogenetics
Further information: PharmacogeneticsLarge bodies of research are devoted to using genetic markers to predict whether patients will respond to SSRIs or have side effects that will cause their discontinuation, although these tests are not yet ready for widespread clinical use.
Versus TCAs
SSRIs are described as 'selective' because they affect only the reuptake pumps responsible for serotonin, as opposed to earlier antidepressants, which affect other monoamine neurotransmitters as well, and as a result, SSRIs have fewer side effects.
There appears to be no significant difference in effectiveness between SSRIs and tricyclic antidepressants, which were the most commonly used class of antidepressants before the development of SSRIs. However, SSRIs have the important advantage that their toxic dose is high, and, therefore, they are much more difficult to use as a means to commit suicide. Further, they have fewer and milder side effects. Tricyclic antidepressants also have a higher risk of serious cardiovascular side effects, which SSRIs lack.
SSRIs act on signal pathways such as cyclic adenosine monophosphate (cAMP) on the postsynaptic neuronal cell, which leads to the release of brain-derived neurotrophic factor (BDNF). BDNF enhances the growth and survival of cortical neurons and synapses.
Pharmacokinetics
SSRIs vary in their pharmacokinetic properties.
| SSRI | FTooltip Bioavailability (%) | VdTooltip Volume of distribution (L/kg) | logPTooltip Partition coefficient | PPBTooltip Plasma protein binding (%) | Major metabolic enzymes (additional) | t1/2Tooltip Elimination half-life (h) | Dose (mg) | Levels (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| Citalopram | 80 | 12 | 3.76 | 80 | CYP2C19, CYP3A4 (CYP2D6) | 35 | 20–40 | 50–110 |
| Escitalopram | 80 | 12 | 3.5 | 56 | CYP3A4, CYP2C19 | 27–32 | 10–20 | 15–80 |
| Fluoxetine | 60–80 | 20–45 | 4.05 | 95 | CYP2D6, CYP2C9 (CYP2C19) | 24–96 | 20–60 | 120–500 |
| Fluvoxamine | 53 | 25 | 2.89 | 77 | CYP2D6 (CYP1A2) | 12–15 | 50–300 | 60–230 |
| Paroxetine | 50–90 | 17 | 3.6 | 95 | CYP2D6 | 21 | 20–50 | 30–120 |
| Sertraline | 80–95 | 20 | 5.1 | 98 | CYP2B6 (CYP2C19, CYP3A4, CYP2D6) | 25–26 | 50–200 | 10–150 |
List of SSRIs
Marketed
Serotonin transporter inhibitors
Antidepressants
- Citalopram (Celexa)
- Escitalopram (Lexapro)
- Fluoxetine (Prozac)
- Fluvoxamine (Luvox)
- Paroxetine (Paxil)
- Sertraline (Zoloft)
 Citalopram Citalopram
|
 Escitalopram Escitalopram
|
 Fluoxetine Fluoxetine
|
 Fluvoxamine Fluvoxamine
|
 Paroxetine Paroxetine
|
 Sertraline Sertraline
| |
Others
- Dapoxetine (Priligy)
 Dapoxetine Dapoxetine
|
Discontinued
Antidepressants
- Indalpine (Upstène)
- Zimelidine (Zelmid)
 Indalpine Indalpine
|
 Zimelidine Zimelidine
|
Never marketed
Antidepressants
- Alaproclate (GEA-654)
- Centpropazine
- Cericlamine (JO-1017)
- Femoxetine (Malexil; FG-4963)
- Ifoxetine (CGP-15210)
- Omiloxetine
- Panuramine (WY-26002)
- Pirandamine (AY-23713)
- Seproxetine ((S)-norfluoxetine)
 Alaproclate Alaproclate
|
 Cericlamine Cericlamine
|
 Femoxetine Femoxetine
|
 Ifoxetine Ifoxetine
|
 Omiloxetine Omiloxetine
|
 Pirandamine Pirandamine
|
 Seproxetine Seproxetine
| |
Related drugs
Although described as SNRIs, duloxetine (Cymbalta), venlafaxine (Effexor), and desvenlafaxine (Pristiq) are in fact relatively selective as serotonin reuptake inhibitors (SRIs). They are about at least 10-fold selective for inhibition of serotonin reuptake over norepinephrine reuptake. The selectivity ratios are approximately 1:30 for venlafaxine, 1:10 for duloxetine, and 1:14 for desvenlafaxine. At low doses, these SNRIs act mostly as SSRIs; only at higher doses do they also prominently inhibit norepinephrine reuptake. Milnacipran (Ixel, Savella) and its stereoisomer levomilnacipran (Fetzima) are the only widely marketed SNRIs that inhibit serotonin and norepinephrine to similar degrees, both with ratios close to 1:1.
Vilazodone (Viibryd) and vortioxetine (Trintellix) are SRIs that also act as modulators of serotonin receptors and are described as serotonin modulators and stimulators (SMS). Vilazodone is a 5-HT1A receptor partial agonist while vortioxetine is a 5-HT1A receptor agonist and 5-HT3 and 5-HT7 receptor antagonist. Litoxetine (SL 81–0385) and lubazodone (YM-992, YM-35995) are similar drugs that were never marketed. They are SRIs and litoxetine is also a 5-HT3 receptor antagonist while lubazodone is also a 5-HT2A receptor antagonist.
History
See also: Development and discovery of SSRI drugsZimelidine was introduced in 1982 and was the first SSRI to be sold. Despite its efficacy, statistically significant increase in cases of Guillain–Barré syndrome among treated patients led to its withdrawal in 1983. Fluoxetine, introduced in 1987 is commonly thought the first SSRI to be marketed.
Controversy
See also: Biopsychiatry controversy and Biological psychiatryA study examining publication of results from FDA-evaluated antidepressants concluded that those with favorable results were much more likely to be published than those with negative results. Furthermore, an investigation of 185 meta-analyses on antidepressants found that 79% of them had authors affiliated in some way to pharmaceutical companies and that they were reluctant to report caveats for antidepressants.
David Healy has argued that warning signs were available for many years prior to regulatory authorities moving to put warnings on antidepressant labels that they might cause suicidal thoughts. At the time these warnings were added, others argued that the evidence for harm remained unpersuasive and others continued to do so after the warnings were added.
In other organisms
SSRIs are common environmental contaminant findings near human settlement.
Veterinary use
An SSRI (fluoxetine) has been approved for veterinary use in treatment of canine separation anxiety.
See also
- List of antidepressants
- Noradrenergic and specific serotonergic antidepressant
- Serotonin releasing agent (SRA)
- Serotonin–norepinephrine reuptake inhibitor (SNRI)
- Serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI)
- Dapoxetine
References
- Barlow DH, durand VM (2009). "Chapter 7: Mood Disorders and Suicide". Abnormal Psychology: An Integrative Approach (Fifth ed.). Belmont, CA: Wadsworth Cengage Learning. p. 239. ISBN 978-0-495-09556-9. OCLC 192055408.
- "Mechanism of Action of Antidepressants" (PDF). Psychopharmacology Bulletin. 36. Summer 2002. S2CID 4937890. Archived from the original (PDF) on 2019-02-28.
- Preskorn SH, Ross R, Stanga CY (2004). "Selective Serotonin Reuptake Inhibitors". In Preskorn SH, Feighner HP, Stanga CY, Ross R (eds.). Antidepressants: Past, Present and Future. Berlin: Springer. pp. 241–262. ISBN 978-3-540-43054-4.
- Rettew D (2022-07-26). "Depression and Serotonin: What the New Review Actually Says". Psychology Today. Retrieved 2024-09-26.
- Kramer P (7 Sep 2011). "In Defense of Antidepressants". The New York Times. Archived from the original on 12 July 2011. Retrieved 13 July 2011.
- ^ Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J (January 2010). "Antidepressant drug effects and depression severity: a patient-level meta-analysis". JAMA. 303 (1): 47–53. doi:10.1001/jama.2009.1943. PMC 3712503. PMID 20051569.
- Pies R (April 2010). "Antidepressants work, sort of – our system of care does not". Journal of Clinical Psychopharmacology. 30 (2): 101–104. doi:10.1097/JCP.0b013e3181d52dea. PMID 20520282. Archived from the original on 2017-09-13. Retrieved 2019-11-08.
- ^ Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C (February 2017). "Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis". BMC Psychiatry. 17 (1): 58. doi:10.1186/s12888-016-1173-2. PMC 5299662. PMID 28178949.
- Medford N, Sierra M, Baker D, David AS (2005). "Understanding and treating depersonalisation disorder". Advances in Psychiatric Treatment. 11 (2): 92–100. doi:10.1192/apt.11.2.92.
- ^ National Collaborating Centre for Mental Health (October 2009). "Depression Quick Reference Guide" (PDF). NICE clinical guidelines 90 and 91. The National Institute for Health and Care Excellence (NICE). Archived from the original (PDF) on September 28, 2013.
- ^ Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration". PLOS Medicine. 5 (2): e45. doi:10.1371/journal.pmed.0050045. PMC 2253608. PMID 18303940.
- Horder J, Matthews P, Waldmann R (June 2010). "Placebo, Prozac and PLoS: significant lessons for psychopharmacology". Journal of Psychopharmacology. 25 (10): 1277–1288. doi:10.1177/0269881110372544. hdl:2108/54719. PMID 20571143. S2CID 10323933.
- Fountoulakis KN, Möller HJ (August 2010). "Efficacy of antidepressants: a re-analysis and re-interpretation of the Kirsch data". The International Journal of Neuropsychopharmacology. 14 (3): 405–412. doi:10.1017/S1461145710000957. PMID 20800012.
- Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ (June 2012). "Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine". Archives of General Psychiatry. 69 (6): 572–579. doi:10.1001/archgenpsychiatry.2011.2044. PMC 3371295. PMID 22393205.
- Hieronymus F, Lisinski A, Näslund J, Eriksson E (2018). "Multiple possible inaccuracies cast doubt on a recent report suggesting selective serotonin reuptake inhibitors to be toxic and ineffective". Acta Neuropsychiatrica. 30 (5): 244–250. doi:10.1017/neu.2017.23. PMID 28718394.
- ^ Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR (April 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. 391 (10128): 1357–1366. doi:10.1016/S0140-6736(17)32802-7. PMC 5889788. PMID 29477251.
- Kirsch I, Moncrieff J (July 2007). "Clinical trials and the response rate illusion". Contemp Clin Trials. 28 (4): 348–351. doi:10.1016/j.cct.2006.10.012. PMID 17182286.
- Moncrieff J, Kirsch I (July 2015). "Empirically derived criteria cast doubt on the clinical significance of antidepressant-placebo differences". Contemp Clin Trials. 43: 60–2. doi:10.1016/j.cct.2015.05.005. PMID 25979317.
The commonly used method of estimating the 'response' to drug treatment in clinical trials of antidepressants (arbitrarily set at a 50% reduction in symptoms), involves the categorisation of continuous data from symptom scales, and therefore does not provide an independent arbiter of clinical significance. Moreover, this method can exaggerate small differences between interventions such as antidepressants and placebo , and statisticians note that it can distort data and should be avoided , . Response rates in double-blind antidepressant trials are typically about 50% in the drug groups and 35% in the placebo groups (e.g., , ). This 15% difference is often defended as clinically significant on the grounds that 15% of depressed people who get better on antidepressants would not have gotten better on placebo. However, a 50% reduction in symptoms is close to the mean and median of drug improvement rates in placebo-controlled antidepressant trials , , and thus near the apex of the distribution curve. Thus, with an SD of 8 in change scores, a 15% difference in response rates is about (an odds ratio of 1.86, a relative risk of 0.77, and an NNT of 7) is exactly what one would expect from a mean 3-point difference in HAM-D scores . Lack of response does not mean that the patient has not improved; it means that the improvement has been less, by as little as one point, than the arbitrary criterion chosen for defining a therapeutic response.
- Hengartner MP (2017). "Methodological Flaws, Conflicts of Interest, and Scientific Fallacies: Implications for the Evaluation of Antidepressants' Efficacy and Harm". Front Psychiatry. 8: 275. doi:10.3389/fpsyt.2017.00275. PMC 5725408. PMID 29270136.
Another common flaw is to report efficacy based on drug-placebo differences in response and remission rates (27). To come at binary constructs such as response and remission, continuous symptom rating scales are dichotomized along arbitrary cut-offs. However, methodologists have vigorously advised against the use of dichotomization (28–30) because it produces, among others, systematically inflated effect sizes (31–33).
- Hetrick SE, McKenzie JE, Bailey AP, Sharma V, Moller CI, Badcock PB, Cox GR, Merry SN, Meader N, et al. (Cochrane Common Mental Disorders Group) (May 2021). "New generation antidepressants for depression in children and adolescents: a network meta-analysis". The Cochrane Database of Systematic Reviews. 2021 (5): CD013674. doi:10.1002/14651858.CD013674.pub2. PMC 8143444. PMID 34029378.
- "Depression in children and young people: identification and management". NICE guideline NG134. The National Institute for Health and Care Excellence (NICE). June 2019. Archived from the original on 2022-12-26. Retrieved 2023-01-16.
- Canton J, Scott KM, Glue P (2012). "Optimal treatment of social phobia: systematic review and meta-analysis". Neuropsychiatric Disease and Treatment. 8: 203–215. doi:10.2147/NDT.S23317. PMC 3363138. PMID 22665997.
- Hedges DW, Brown BL, Shwalb DA, Godfrey K, Larcher AM (January 2007). "The efficacy of selective serotonin reuptake inhibitors in adult social anxiety disorder: a meta-analysis of double-blind, placebo-controlled trials". J Psychopharmacol. 21 (1): 102–11. doi:10.1177/0269881106065102. PMID 16714326. S2CID 21795838.
- Alexander W (January 2012). "Pharmacotherapy for Post-traumatic Stress Disorder In Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents". P & T. 37 (1): 32–38. PMC 3278188. PMID 22346334.
- ^ "www.nice.org.uk" (PDF). Archived from the original (PDF) on 2012-10-21. Retrieved 2013-02-20.
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, Antony MM, Bouchard S, Brunet A, Flament M, Grigoriadis S, Mendlowitz S, O'Connor K, Rabheru K, Richter PM, Robichaud M, Walker JR (2014-07-02). "Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders". BMC Psychiatry. 14 (Suppl 1): S1. doi:10.1186/1471-244X-14-S1-S1. PMC 4120194. PMID 25081580.
- "Obsessive-compulsive disorder: Core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder" (PDF). November 2005. Archived from the original (PDF) on 2008-12-06. Retrieved 2013-02-24.
- Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, Kerse N, Macgillivray S (July 2009). Arroll B (ed.). "Antidepressants versus placebo for depression in primary care". The Cochrane Database of Systematic Reviews. 2009 (3): CD007954. doi:10.1002/14651858.CD007954. PMC 10576545. PMID 19588448.
- Busko M (28 February 2008). "Review Finds SSRIs Modestly Effective in Short-Term Treatment of OCD". Medscape. Archived from the original on April 13, 2013.
- Fineberg NA, Brown A, Reghunandanan S, Pampaloni I (September 2012). "Evidence-based pharmacotherapy of obsessive-compulsive disorder". The International Journal of Neuropsychopharmacology. 15 (8): 1173–1191. doi:10.1017/S1461145711001829. hdl:2299/216. PMID 22226028.
- "Sertraline prescribing information" (PDF). Archived (PDF) from the original on 2015-06-16. Retrieved 2015-01-30.
- "Paroxetine prescribing information" (PDF). Archived (PDF) from the original on 2015-02-19. Retrieved 2015-01-30.
- Batelaan NM, Van Balkom AJ, Stein DJ (April 2012). "Evidence-based pharmacotherapy of panic disorder: an update". The International Journal of Neuropsychopharmacology. 15 (3): 403–415. doi:10.1017/S1461145711000800. PMID 21733234.
- Asnis GM, Hameedi FA, Goddard AW, Potkin SG, Black D, Jameel M, Desagani K, Woods SW (August 2001). "Fluvoxamine in the treatment of panic disorder: a multi-center, double-blind, placebo-controlled study in outpatients". Psychiatry Research. 103 (1): 1–14. doi:10.1016/s0165-1781(01)00265-7. PMID 11472786. S2CID 40412606.
- Bighelli I, Castellazzi M, Cipriani A, Girlanda F, Guaiana G, Koesters M, Turrini G, Furukawa TA, Barbui C (April 2018). "Antidepressants versus placebo for panic disorder in adults". The Cochrane Database of Systematic Reviews. 2018 (4): CD010676. doi:10.1002/14651858.CD010676.pub2. PMC 6494573. PMID 29620793.
- ^ "Eating disorders in over 8s: management" (PDF). Clinical guideline . The National Institute for Health and Care Excellence (NICE). January 2004. Archived (PDF) from the original on 2014-03-27. Retrieved 2013-03-02.
- ^ "Practice guideline for the treatment of patients with eating disorders". National Guideline Clearinghouse. U.S. Department of Health and Human Services. Archived from the original on 2013-05-25.
- Flament MF, Bissada H, Spettigue W (March 2012). "Evidence-based pharmacotherapy of eating disorders". The International Journal of Neuropsychopharmacology. 15 (2): 189–207. doi:10.1017/S1461145711000381. PMID 21414249.
- Legg LA, Rudberg AS, Hua X, Wu S, Hackett ML, Tilney R, Lindgren L, Kutlubaev MA, Hsieh CF, Barugh AJ, Hankey GJ, Lundström E, Dennis M, Mead GE (2021-11-15). "Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery". The Cochrane Database of Systematic Reviews. 2021 (11): CD009286. doi:10.1002/14651858.CD009286.pub4. ISSN 1469-493X. PMC 8592088. PMID 34780067.
- Waldinger MD (November 2007). "Premature ejaculation: state of the art". The Urologic Clinics of North America. 34 (4): 591–599, vii–viii. doi:10.1016/j.ucl.2007.08.011. PMID 17983899.
- Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA (January 2010). "The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments". Pharmaceuticals. 3 (1): 19–41. doi:10.3390/ph3010019. PMC 3991019. PMID 27713241.
- Higgins A, Nash M, Lynch AM (September 2010). "Antidepressant-associated sexual dysfunction: impact, effects, and treatment". Drug, Healthcare and Patient Safety. 2: 141–150. doi:10.2147/DHPS.S7634. PMC 3108697. PMID 21701626.
- Romero-Martínez Á, Murciano-Martí S, Moya-Albiol L (May 2019). "Is Sertraline a Good Pharmacological Strategy to Control Anger? Results of a Systematic Review". Behavioral Sciences. 9 (5): 57. doi:10.3390/bs9050057. PMC 6562745. PMID 31126061.
- Stahl SM, Lonnen AJ (2011). "The Mechanism of Drug-induced Akathsia". CNS Spectrums. PMID 21406165.
- Lane RM (1998). "SSRI-induced extrapyramidal side-effects and akathisia: implications for treatment". Journal of Psychopharmacology. 12 (2): 192–214. doi:10.1177/026988119801200212. PMID 9694033. S2CID 20944428.
- Koliscak LP, Makela EH (2009). "Selective serotonin reuptake inhibitor-induced akathisia". Journal of the American Pharmacists Association. 49 (2): e28–36, quiz e37–38. doi:10.1331/JAPhA.2009.08083. PMID 19289334.
- Leo RJ (1996). "Movement disorders associated with the serotonin selective reuptake inhibitors". The Journal of Clinical Psychiatry. 57 (10): 449–454. doi:10.4088/jcp.v57n1002. PMID 8909330.
- Bahrick AS (2008). "Persistence of Sexual Dysfunction Side Effects after Discontinuation of Antidepressant Medications: Emerging Evidence". The Open Psychology Journal. 1: 42–50. doi:10.2174/1874350100801010042. Archived from the original on 2021-04-15. Retrieved 2021-04-15.
- Taylor MJ, Rudkin L, Bullemor-Day P, Lubin J, Chukwujekwu C, Hawton K (May 2013). "Strategies for managing sexual dysfunction induced by antidepressant medication". The Cochrane Database of Systematic Reviews. 5 (5): CD003382. doi:10.1002/14651858.CD003382.pub3. PMID 23728643.
- Kennedy SH, Rizvi S (April 2009). "Sexual dysfunction, depression, and the impact of antidepressants". Journal of Clinical Psychopharmacology. 29 (2): 157–164. doi:10.1097/jcp.0b013e31819c76e9. PMID 19512977. S2CID 739831.
- Gitlin MJ (September 1994). "Psychotropic medications and their effects on sexual function: diagnosis, biology, and treatment approaches". The Journal of Clinical Psychiatry. 55 (9): 406–413. PMID 7929021.
- Balon R (2006). "SSRI-Associated Sexual Dysfunction". The American Journal of Psychiatry. 163 (9): 1504–1509, quiz 1664. doi:10.1176/appi.ajp.163.9.1504. PMID 16946173.
- Wilson TK, Tripp J (17 January 2023). "Buspirone". StatPearls. PMID 30285372. Archived from the original on 11 August 2020. Retrieved 4 August 2024.
- Trinchieri M, Trinchieri M, Perletti G, Magri V, Stamatiou K, Cai T, Montanari E, Trinchieri A (August 2021). "Erectile and Ejaculatory Dysfunction Associated with Use of Psychotropic Drugs: A Systematic Review". The Journal of Sexual Medicine. 18 (8): 1354–1363. doi:10.1016/j.jsxm.2021.05.016. PMID 34247952.
Buspirone, a non-benzodiazepine anxiolytic, have even demonstrated enhancement of sexual function in certain individuals. For this reason, they have been proposed as augmentation agents (antidotes) or substitution agents in patients with emerging sexual dysfunction after treatment with antidepressants.
- Montejo AL, Prieto N, de Alarcón R, Casado-Espada N, de la Iglesia J, Montejo L (October 2019). "Management Strategies for Antidepressant-Related Sexual Dysfunction: A Clinical Approach". Journal of Clinical Medicine. 8 (10): 1640. doi:10.3390/jcm8101640. PMC 6832699. PMID 31591339.
- Serretti A, Chiesa A (June 2009). "Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis". Journal of Clinical Psychopharmacology. 29 (3): 259–266. doi:10.1097/JCP.0b013e3181a5233f. PMID 19440080. S2CID 1663570.
- Clayton AH (2003). "Antidepressant-Associated Sexual Dysfunction: A Potentially Avoidable Therapeutic Challenge". Primary Psychiatry. 10 (1): 55–61. Archived from the original on 2020-06-04. Retrieved 2013-02-19.
- Kanaly KA, Berman JR (December 2002). "Sexual side effects of SSRI medications: potential treatment strategies for SSRI-induced female sexual dysfunction". Current Women's Health Reports. 2 (6): 409–416. PMID 12429073.
- Xu J, He K, Zhou Y, Zhao L, Lin Y, Huang Z, Xie N, Yue J, Tang Y (2022). "The effect of SSRIs on Semen quality: A systematic review and meta-analysis". Frontiers in Pharmacology. 13: 911489. doi:10.3389/fphar.2022.911489. PMC 9519136. PMID 36188547.
- Koyuncu H, Serefoglu EC, Ozdemir AT, Hellstrom WJ (September 2012). "Deleterious effects of selective serotonin reuptake inhibitor treatment on semen parameters in patients with lifelong premature ejaculation". International Journal of Impotence Research. 24 (5): 171–173. doi:10.1038/ijir.2012.12. PMID 22573230.
- Scherzer ND, Reddy AG, Le TV, Chernobylsky D, Hellstrom WJ (April 2019). "Unintended Consequences: A Review of Pharmacologically-Induced Priapism". Sexual Medicine Reviews. 7 (2): 283–292. doi:10.1016/j.sxmr.2018.09.002. PMID 30503727. S2CID 54621798.
- Jannini TB, Lorenzo GD, Bianciardi E, et al. (2022). "Off-label Uses of Selective Serotonin Reuptake Inhibitors (SSRIs)". Curr Neuropharmacol (Review). 20 (4): 693–712. doi:10.2174/1570159X19666210517150418. PMC 9878961. PMID 33998993.
- ^ Tarchi L, Merola GP, Baccaredda-Boy O, et al. (June 2023). "Selective serotonin reuptake inhibitors, post-treatment sexual dysfunction and persistent genital arousal disorder: A systematic review". Pharmacoepidemiol Drug Saf (Review). 32 (10): 1053–1067. doi:10.1002/pds.5653. hdl:2158/1317239. PMID 37294623. S2CID 259126886. Archived from the original on 2023-07-20. Retrieved 2023-08-15.
- ^ Healy D, Bahrick A, Bak M, Barbato A, Calabrò RS, Chubak BM, Cosci F, Csoka AB, D'Avanzo B, Diviccaro S, Giatti S, Goldstein I, Graf H, Hellstrom WJ, Irwig MS, Jannini EA, Janssen PK, Khera M, Kumar MT, Le Noury J, Lew-Starowicz M, Linden DE, Lüning C, Mangin D, Melcangi RC, Rodríguez OW, Panicker JN, Patacchini A, Pearlman AM, Pukall CF, Raj S, Reisman Y, Rubin RS, Schreiber R, Shipko S, Vašečková B, Waraich A (1 January 2022). "Diagnostic criteria for enduring sexual dysfunction after treatment with antidepressants, finasteride and isotretinoin". The International Journal of Risk & Safety in Medicine. 33 (1): 65–76. doi:10.3233/JRS-210023. PMC 8925105. PMID 34719438.
- ^ Chinchilla Alfaro K, van Hunsel F, Ekhart C (April 2022). "Persistent sexual dysfunction after SSRI withdrawal: a scoping review and presentation of 86 cases from the Netherlands". Expert Opinion on Drug Safety (Review). 21 (4): 553–561. doi:10.1080/14740338.2022.2007883. PMID 34791958. S2CID 244347777.
- Marks S (July 2023). "A clinical review of antidepressants, their sexual side-effects, post-SSRI sexual dysfunction, and serotonin syndrome" (PDF). Br J Nurs. 32 (14): 678–682. doi:10.12968/bjon.2023.32.14.678. PMID 37495413. S2CID 260202178. Archived (PDF) from the original on 2024-03-22. Retrieved 2024-03-22.
- ^ Bala A, Nguyen HM, Hellstrom WJ (January 2018). "Post-SSRI Sexual Dysfunction: A Literature Review". Sexual Medicine Reviews (Review). 6 (1): 29–34. doi:10.1016/j.sxmr.2017.07.002. PMID 28778697.
There is still no definitive treatment for PSSD. Low-power laser irradiation and phototherapy have shown some promising results.
- ^ Peleg LC, Rabinovitch D, Lavie Y, et al. (January 2022). "Post-SSRI Sexual Dysfunction (PSSD): Biological Plausibility, Symptoms, Diagnosis, and Presumed Risk Factors". Sex Med Rev (Review). 10 (1): 91–98. doi:10.1016/j.sxmr.2021.07.001. PMID 34627736. S2CID 238580777.
- Giatti S, Diviccaro S, Panzica G, Melcangi RC (August 2018). "Post-finasteride syndrome and post-SSRI sexual dysfunction: two sides of the same coin?". Endocrine (Review). 61 (2): 180–193. doi:10.1007/s12020-018-1593-5. PMID 29675596. S2CID 4974636.
- Rothmore J (April 2020). "Antidepressant-induced sexual dysfunction". Med J Aust (Review). 212 (7): 329–334. doi:10.5694/mja2.50522. PMID 32172535. S2CID 212728659.
- Pirani Y, Delgado-Ron JA, Marinho P, Gupta A, Grey E, Watt S, MacKinnon KR, Salway T (2024-09-20). "Frequency of self-reported persistent post-treatment genital hypoesthesia among past antidepressant users: a cross-sectional survey of sexual and gender minority youth in Canada and the US". Social Psychiatry and Psychiatric Epidemiology. doi:10.1007/s00127-024-02769-0. ISSN 1433-9285.
- PRAC recommendations on signals: Adopted at the 13-16 May 2019 PRAC meeting (PDF). European Medicines Agency. 11 June 2019. p. 5. Archived (PDF) from the original on 20 July 2023. Retrieved 19 July 2023.
- "SSRIs, SNRIs: risk of persistent sexual dysfunction". Reactions Weekly. 1838 (5). Springer: 5. 16 January 2021. doi:10.1007/s40278-021-89324-7. S2CID 231669986.
- "Csoka v. FDA". Public Citizen. Retrieved 2024-09-15.
- "FDA Sued Over Inaction on Citizen Petition". Public Citizen. 2024-05-20. Retrieved 2024-09-15.
- Marazziti D, Mucci F, Tripodi B, Carbone MG, Muscarella A, Falaschi V, Baroni S (April 2019). "Emotional Blunting, Cognitive Impairment, Bone Fractures, and Bleeding as Possible Side Effects of Long-Term Use of SSRIs". Clin Neuropsychiatry. 16 (2): 75–85. PMC 8650205. PMID 34908941.
- ^ Ma H, Cai M, Wang H (2021). "Emotional Blunting in Patients With Major Depressive Disorder: A Brief Non-systematic Review of Current Research". Front Psychiatry. 12: 792960. doi:10.3389/fpsyt.2021.792960. PMC 8712545. PMID 34970173.
- Moncrieff J (October 2015). "Antidepressants: misnamed and misrepresented". World Psychiatry. 14 (3): 302–303. doi:10.1002/wps.20243. PMC 4592647. PMID 26407780.
- Corruble E, de Bodinat C, Belaïdi C, Goodwin GM (November 2013). "Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: a 24-wk randomized, controlled, double-blind trial". Int J Neuropsychopharmacol. 16 (10): 2219–2234. doi:10.1017/S1461145713000679. PMID 23823799.
- Fagiolini A, Florea I, Loft H, Christensen MC (March 2021). "Effectiveness of Vortioxetine on Emotional Blunting in Patients with Major Depressive Disorder with inadequate response to SSRI/SNRI treatment". J Affect Disord. 283: 472–479. doi:10.1016/j.jad.2020.11.106. hdl:11365/1137950. PMID 33516560. S2CID 228877905.
- Costagliola C, Parmeggiani F, Semeraro F, Sebastiani A (December 2008). "Selective serotonin reuptake inhibitors: a review of its effects on intraocular pressure". Current Neuropharmacology. 6 (4): 293–310. doi:10.2174/157015908787386104. PMC 2701282. PMID 19587851.
- Lochhead J (September 2015). "SSRI-associated optic neuropathy". Eye. 29 (9): 1233–1235. doi:10.1038/eye.2015.119. PMC 4565945. PMID 26139049.
- Oh SW, Kim J, Myung SK, Hwang SS, Yoon DH (Mar 20, 2014). "Antidepressant Use and Risk of Coronary Heart Disease: Meta-Analysis of Observational Studies". British Journal of Clinical Pharmacology. 78 (4): 727–737. doi:10.1111/bcp.12383. PMC 4239967. PMID 24646010.
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, Mogun H, Levin R, Kowal M, Setoguchi S, Hernández-Díaz S (2014). "Antidepressant Use in Pregnancy and the Risk of Cardiac Defects". New England Journal of Medicine. 370 (25): 2397–2407. doi:10.1056/NEJMoa1312828. PMC 4062924. PMID 24941178.
- Goldberg RJ (1998). "Selective serotonin reuptake inhibitors: infrequent medical adverse effects". Archives of Family Medicine. 7 (1): 78–84. doi:10.1001/archfami.7.1.78. PMID 9443704.
- FDA (December 2018). "FDA Drug Safety". FDA. Archived from the original on 2020-10-10. Retrieved 2019-12-16.
- Citalopram and escitalopram: QT interval prolongation – new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings Archived 2013-03-06 at the Wayback Machine. From Medicines and Healthcare products Regulatory Agency. Article date: December 2011
- "Clinical and ECG Effects of Escitalopram Overdose" (PDF). Archived (PDF) from the original on 2013-10-21. Retrieved 2012-09-23.
- Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V (Jun 1999). "Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any?". Current Medicinal Chemistry. 6 (6): 469–480. doi:10.2174/0929867306666220330184544. PMID 10213794. S2CID 28057842.
- "Deciphering the Connection of Serotonin to Degenerative Mitral Valve Regurgitation - Advances in Cardiology and Heart Surgery". NewYork-Presbyterian. Archived from the original on 2024-02-12. Retrieved 2024-02-12.
- Castillero E, Fitzpatrick E, Keeney SJ, D'Angelo AM, Pressly BB, Simpson MT, Kurade M, Erwin WC, Moreno V, Camillo C, Shukla HJ, Inamdar VV, Aghali A, Grau JB, Salvati E (2023-01-04). "Decreased serotonin transporter activity in the mitral valve contributes to progression of degenerative mitral regurgitation". Science Translational Medicine. 15 (677): eadc9606. doi:10.1126/scitranslmed.adc9606. ISSN 1946-6234. PMC 9896655. PMID 36599005.
- "Serotonin can potentially accelerate degenerative mitral regurgitation, study says". News-Medical. 2023-01-29. Archived from the original on 2024-02-12. Retrieved 2024-02-12.
- Andrade C, Sharma E (September 2016). "Serotonin Reuptake Inhibitors and Risk of Abnormal Bleeding". The Psychiatric Clinics of North America. 39 (3): 413–426. doi:10.1016/j.psc.2016.04.010. PMID 27514297.
- ^ Weinrieb RM, Auriacombe M, Lynch KG, Lewis JD (March 2005). "Selective serotonin re-uptake inhibitors and the risk of bleeding". Expert Opinion on Drug Safety. 4 (2): 337–344. doi:10.1517/14740338.4.2.337. PMID 15794724. S2CID 46551382.
- ^ Taylor D, Carol P, Shitij K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97969-3.
- ^ Andrade C, Sandarsh S, Chethan KB, Nagesh KS (December 2010). "Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms". The Journal of Clinical Psychiatry. 71 (12): 1565–1575. doi:10.4088/JCP.09r05786blu. PMID 21190637.
- ^ de Abajo FJ, García-Rodríguez LA (July 2008). "Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents". Archives of General Psychiatry. 65 (7): 795–803. doi:10.1001/archpsyc.65.7.795. PMID 18606952.
- Hackam DG, Mrkobrada M (October 2012). "Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis". Neurology. 79 (18): 1862–1865. doi:10.1212/WNL.0b013e318271f848. PMID 23077009. S2CID 11941911.
- Serebruany VL (February 2006). "Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something?". The American Journal of Medicine. 119 (2): 113–116. doi:10.1016/j.amjmed.2005.03.044. PMID 16443409.
- Halperin D, Reber G (2007). "Influence of antidepressants on hemostasis". Dialogues in Clinical Neuroscience. 9 (1): 47–59. doi:10.31887/DCNS.2007.9.1/dhalperin. PMC 3181838. PMID 17506225.
- de Abajo FJ (May 2011). "Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients". Drugs & Aging. 28 (5): 345–367. doi:10.2165/11589340-000000000-00000. PMID 21542658. S2CID 116561324.
- Eom CS, Lee HK, Ye S, Park SM, Cho KH (May 2012). "Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis". Journal of Bone and Mineral Research. 27 (5): 1186–1195. doi:10.1002/jbmr.1554. PMID 22258738.
- Bruyère O, Reginster JY (February 2015). "Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome". Endocrine. 48 (1): 65–68. doi:10.1007/s12020-014-0357-0. PMID 25091520. S2CID 32286954. Archived from the original on 2020-10-10. Retrieved 2019-07-01.
- Hant FN, Bolster MB (April 2016). "Drugs that may harm bone: Mitigating the risk". Cleveland Clinic Journal of Medicine. 83 (4): 281–288. doi:10.3949/ccjm.83a.15066. PMID 27055202.
- Fernandes BS, Hodge JM, Pasco JA, Berk M, Williams LJ (January 2016). "Effects of Depression and Serotonergic Antidepressants on Bone: Mechanisms and Implications for the Treatment of Depression". Drugs & Aging. 33 (1): 21–25. doi:10.1007/s40266-015-0323-4. PMID 26547857. S2CID 7648524.
- Nyandege AN, Slattum PW, Harpe SE (April 2015). "Risk of fracture and the concomitant use of bisphosphonates with osteoporosis-inducing medications". The Annals of Pharmacotherapy. 49 (4): 437–447. doi:10.1177/1060028015569594. PMID 25667198. S2CID 20622369.
- ^ Warden SJ, Fuchs RK (October 2016). "Do Selective Serotonin Reuptake Inhibitors (SSRIs) Cause Fractures?". Current Osteoporosis Reports. 14 (5): 211–218. doi:10.1007/s11914-016-0322-3. PMID 27495351. S2CID 5610316.
- Winterhalder L, Eser P, Widmer J, Villiger PM, Aeberli D (December 2012). "Changes in volumetric BMD of radius and tibia upon antidepressant drug administration in young depressive patients". Journal of Musculoskeletal & Neuronal Interactions. 12 (4): 224–229. PMID 23196265.
- Garrett AR, Hawley JS (April 2018). "SSRI-associated bruxism: A systematic review of published case reports". Neurology. Clinical Practice. 8 (2): 135–141. doi:10.1212/CPJ.0000000000000433. PMC 5914744. PMID 29708207.
- Prisco V, Iannaccone T, Di Grezia G (2017-04-01). "Use of buspirone in selective serotonin reuptake inhibitor-induced sleep bruxism". European Psychiatry. Abstract of the 25th European Congress of Psychiatry. 41: S855. doi:10.1016/j.eurpsy.2017.01.1701. ISSN 0924-9338. S2CID 148816505. Archived from the original on 2020-10-10. Retrieved 2020-04-18.
- Albayrak Y, Ekinci O (2011). "Duloxetine-induced nocturnal bruxism resolved by buspirone: case report". Clinical Neuropharmacology. 34 (4): 137–138. doi:10.1097/WNF.0b013e3182227736. PMID 21768799.
- Volpi-Abadie J, Kaye AM, Kaye AD (2013). "Serotonin syndrome". The Ochsner Journal. 13 (4): 533–540. PMC 3865832. PMID 24358002.
- Boyer EW, Shannon M (March 2005). "The serotonin syndrome". The New England Journal of Medicine. 352 (11): 1112–1120. doi:10.1056/nejmra041867. PMID 15784664. S2CID 37959124.
- Orlova Y, Rizzoli P, Loder E (May 2018). "Association of Coprescription of Triptan Antimigraine Drugs and Selective Serotonin Reuptake Inhibitor or Selective Norepinephrine Reuptake Inhibitor Antidepressants With Serotonin Syndrome". JAMA Neurology. 75 (5): 566–572. doi:10.1001/jamaneurol.2017.5144. PMC 5885255. PMID 29482205.
- Ferri FF (2016). Ferri's Clinical Advisor 2017: 5 Books in 1. Elsevier Health Sciences. pp. 1154–1155. ISBN 978-0-323-44838-3. Archived from the original on 2023-01-14. Retrieved 2021-01-26.
- ^ Stone MB, Jones ML (2006-11-17). "Clinical review: relationship between antidepressant drugs and suicidal behavior in adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Archived (PDF) from the original on 2007-03-16. Retrieved 2007-09-22.
- Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Archived (PDF) from the original on 2007-03-16. Retrieved 2007-09-22.
- Olfson M, Marcus SC, Shaffer D (August 2006). "Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study". Archives of General Psychiatry. 63 (8): 865–872. doi:10.1001/archpsyc.63.8.865. PMID 16894062.
- Hammad TA (2004-08-16). "Review and evaluation of clinical data. Relationship between psychiatric drugs and pediatric suicidal behavior" (PDF). FDA. pp. 42, 115. Archived (PDF) from the original on 2008-06-25. Retrieved 2008-05-29.
- "Antidepressant Use in Children, Adolescents, and Adults". U.S. Food and Drug Administration. Archived from the original on 7 January 2017.
- "FDA Medication Guide for Antidepressants". Food and Drug Administration. Archived from the original on 2014-08-18. Retrieved 2014-06-05.
- ^ Cox GR, Callahan P, Churchill R, Hunot V, Merry SN, Parker AG, Hetrick SE (November 2014). "Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents". The Cochrane Database of Systematic Reviews. 2014 (11): CD008324. doi:10.1002/14651858.CD008324.pub3. PMC 8556660. PMID 25433518.
- "Overview | Depression in adults: recognition and management | Guidance | NICE". www.nice.org.uk. 28 October 2009. Archived from the original on 31 May 2022. Retrieved 30 May 2022.
- Tauscher-Wisniewski S, Nilsson M, Caldwell C, Plewes J, Allen AJ (October 2007). "Meta-analysis of aggression and/or hostility-related events in children and adolescents treated with fluoxetine compared with placebo". Journal of Child and Adolescent Psychopharmacology. 17 (5): 713–718. doi:10.1089/cap.2006.0138. PMID 17979590.
- Gibbons RD, Hur K, Bhaumik DK, Mann JJ (November 2006). "The relationship between antidepressant prescription rates and rate of early adolescent suicide". The American Journal of Psychiatry. 163 (11): 1898–1904. doi:10.1176/appi.ajp.163.11.1898. PMID 17074941. S2CID 2390497.
- "Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants" (PDF). MHRA. 2004-12-01. Archived (PDF) from the original on 2008-02-28. Retrieved 2007-09-25.
- "Selective Serotonin Reuptake Inhibitors (SSRIs): Overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents including a summary of available safety and efficacy data". MHRA. 2005-09-29. Archived from the original on 2008-08-02. Retrieved 2008-05-29.
- Gunnell D, Saperia J, Ashby D (February 2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review". BMJ. 330 (7488): 385. doi:10.1136/bmj.330.7488.385. PMC 549105. PMID 15718537.
- Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P, Hutton B (February 2005). "Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials". BMJ. 330 (7488): 396. doi:10.1136/bmj.330.7488.396. PMC 549110. PMID 15718539.
- Rihmer Z, Akiskal H (August 2006). "Do antidepressants t(h)reat(en) depressives? Toward a clinically judicious formulation of the antidepressant-suicidality FDA advisory in light of declining national suicide statistics from many countries". Journal of Affective Disorders. 94 (1–3): 3–13. doi:10.1016/j.jad.2006.04.003. PMID 16712945.
- Hall WD, Lucke J (2006). "How have the selective serotonin reuptake inhibitor antidepressants affected suicide mortality?". The Australian and New Zealand Journal of Psychiatry. 40 (11–12): 941–950. doi:10.1111/j.1440-1614.2006.01917.x. PMID 17054562.
- Martínez-Aguayo JC, Arancibia M, Concha S, Madrid E (2016). "Ten years after the FDA black box warning for antidepressant drugs: A critical narrative review". Archives of Clinical Psychiatry. 43 (3): 60–66. doi:10.1590/0101-60830000000086.
- ^ Maslej MM, Bolker BM, Russell MJ, Eaton K, Durisko Z, Hollon SD, Swanson GM, Thomson JA, Mulsant BH, Andrews PW (2017). "The Mortality and Myocardial Effects of Antidepressants Are Moderated by Preexisting Cardiovascular Disease: A Meta-Analysis". Psychother Psychosom. 86 (5): 268–282. doi:10.1159/000477940. PMID 28903117. S2CID 4830115.
- Malm H (December 2012). "Prenatal exposure to selective serotonin reuptake inhibitors and infant outcome". Therapeutic Drug Monitoring. 34 (6): 607–614. doi:10.1097/FTD.0b013e31826d07ea. PMID 23042258. S2CID 22875385.
- Rahimi R, Nikfar S, Abdollahi M (2006). "Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials". Reproductive Toxicology. 22 (4): 571–575. Bibcode:2006RepTx..22..571R. doi:10.1016/j.reprotox.2006.03.019. PMID 16720091.
- ^ Nikfar S, Rahimi R, Hendoiee N, Abdollahi M (2012). "Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: A systematic review and updated meta-analysis". DARU Journal of Pharmaceutical Sciences. 20 (1): 75. doi:10.1186/2008-2231-20-75. PMC 3556001. PMID 23351929.
- Eke AC, Saccone G, Berghella V (November 2016). "Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis". BJOG. 123 (12): 1900–1907. doi:10.1111/1471-0528.14144. PMC 9987176. PMID 27239775.
- Dubovicky M, Belovicova K, Csatlosova K, Bogi E (September 2017). "Risks of using SSRI / SNRI antidepressants during pregnancy and lactation". Interdisciplinary Toxicology. 10 (1): 30–34. doi:10.1515/intox-2017-0004. PMC 6096863. PMID 30123033.
- Einarson TR, Kennedy D, Einarson A (2012). "Do findings differ across research design? The case of antidepressant use in pregnancy and malformations". Journal of Population Therapeutics and Clinical Pharmacology. 19 (2): e334–348. PMID 22946124.
- Riggin L, Frankel Z, Moretti M, Pupco A, Koren G (April 2013). "The fetal safety of fluoxetine: a systematic review and meta-analysis". Journal of Obstetrics and Gynaecology Canada. 35 (4): 362–369. doi:10.1016/S1701-2163(15)30965-8. PMID 23660045.
- Koren G, Nordeng HM (February 2013). "Selective serotonin reuptake inhibitors and malformations: case closed?". Seminars in Fetal & Neonatal Medicine. 18 (1): 19–22. doi:10.1016/j.siny.2012.10.004. PMID 23228547.
- "Breastfeeding Update: SDCBC's quarterly newsletter". Breastfeeding.org. Archived from the original on February 25, 2009. Retrieved 2010-07-10.
- "Using Antidepressants in Breastfeeding Mothers". kellymom.com. Archived from the original on 2010-09-23. Retrieved 2010-07-10.
- Gentile S, Rossi A, Bellantuono C (2007). "SSRIs during breastfeeding: spotlight on milk-to-plasma ratio". Archives of Women's Mental Health. 10 (2): 39–51. doi:10.1007/s00737-007-0173-0. PMID 17294355. S2CID 757921.
- Fenger-Grøn J, Thomsen M, Andersen KS, Nielsen RG (September 2011). "Paediatric outcomes following intrauterine exposure to serotonin reuptake inhibitors: a systematic review". Danish Medical Bulletin. 58 (9): A4303. PMID 21893008.
- Kieviet N, Dolman KM, Honig A (2013). "The use of psychotropic medication during pregnancy: how about the newborn?". Neuropsychiatric Disease and Treatment. 9: 1257–1266. doi:10.2147/NDT.S36394. PMC 3770341. PMID 24039427.
- Persistent Newborn Pulmonary Hypertension at eMedicine
- Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Ross LE (2014). "Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis". BMJ. 348: f6932. doi:10.1136/bmj.f6932. PMC 3898424. PMID 24429387.
- 't Jong GW, Einarson T, Koren G, Einarson A (November 2012). "Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review". Reproductive Toxicology. 34 (3): 293–297. Bibcode:2012RepTx..34..293T. doi:10.1016/j.reprotox.2012.04.015. PMID 22564982.
- Gentile S (August 2015). "Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods?". Journal of Affective Disorders. 182: 132–137. doi:10.1016/j.jad.2015.04.048. PMID 25985383.
- Hviid A, Melbye M, Pasternak B (December 2013). "Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism". The New England Journal of Medicine. 369 (25): 2406–2415. doi:10.1056/NEJMoa1301449. PMID 24350950. S2CID 9064353.
- ^ Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, Weissman M, Wickramaratne P, Artama M, Gingrich JA, Sourander A, et al. (May 2016). "Gestational Exposure to Selective Serotonin Reuptake Inhibitors and Offspring Psychiatric Disorders: A National Register-Based Study". Journal of the American Academy of Child and Adolescent Psychiatry. 55 (5): 359–366. doi:10.1016/j.jaac.2016.02.013. PMC 4851729. PMID 27126849.
- Chris Aiken: Antidepressants in Bipolar II Disorder, May 14, 2019. In: psychiatrictimes.com
- Gitlin MJ (December 2018). "Antidepressants in bipolar depression: an enduring controversy". International Journal of Bipolar Disorders. 6 (1): 25. doi:10.1186/s40345-018-0133-9. PMC 6269438. PMID 30506151.
- Viktorin A, Lichtenstein P, Thase ME, Larsson H, Lundholm C, Magnusson PK, Landén M (October 2014). "The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer". The American Journal of Psychiatry. 171 (10): 1067–1073. doi:10.1176/appi.ajp.2014.13111501. hdl:10616/42159. PMID 24935197. S2CID 25152608.
- Walkup J, Labellarte M (2001). "Complications of SSRI treatment". Journal of Child and Adolescent Psychopharmacology. 11 (1): 1–4. doi:10.1089/104454601750143320. PMID 11322738.
- Ener RA, Meglathery SB, Van Decker WA, Gallagher RM (March 2003). "Serotonin syndrome and other serotonergic disorders". Pain Medicine. 4 (1): 63–74. doi:10.1046/j.1526-4637.2003.03005.x. PMID 12873279.
- Boyer EW, Shannon M (March 2005). "The serotonin syndrome". The New England Journal of Medicine. 352 (11): 1112–1120. doi:10.1056/NEJMra041867. PMID 15784664. S2CID 37959124.
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P (May 2011). "Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans". Proceedings of the National Academy of Sciences of the United States of America. 108 (22): 9262–9267. doi:10.1073/pnas.1104836108. PMC 3107316. PMID 21518864.
- Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill Professional. ISBN 978-0-07-162442-8.
- Ciraulo DA, Shader RI (2011). Ciraulo DA, Shader RI (eds.). Pharmacotherapy of Depression (2nd ed.). Springer. p. 49. doi:10.1007/978-1-60327-435-7. ISBN 978-1-60327-435-7.
- ^ Wyska E (October 2019). "Pharmacokinetic considerations for current state-of-the-art antidepressants". Expert Opin Drug Metab Toxicol. 15 (10): 831–847. doi:10.1080/17425255.2019.1669560. PMID 31526279.
- Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brøsen K (1996). "Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine". European Journal of Clinical Pharmacology. 51 (1): 73–78. doi:10.1007/s002280050163. PMID 8880055. S2CID 19802446.
- Overholser BR, Foster DR (September 2011). "Opioid pharmacokinetic drug-drug interactions". The American Journal of Managed Care. 17 (Suppl 11): S276–287. PMID 21999760.
- "Paroxetine hydrochloride – Drug Summary". Physicians' Desk Reference, LLC. Archived from the original on 2018-09-28. Retrieved 2018-09-17.
- Smith HS (July 2009). "Opioid metabolism". Mayo Clinic Proceedings. 84 (7): 613–624. doi:10.4065/84.7.613. PMC 2704133. PMID 19567715.
- Wiley K, Regan A, McIntyre P (August 2017). "Immunisation and pregnancy – who, what, when and why?". Australian Prescriber. 40 (4): 122–124. doi:10.18773/austprescr.2017.046. PMC 5601969. PMID 28947846.
- Weaver JM (2013). "New FDA black box warning for codeine: how will this affect dentists?". Anesthesia Progress. 60 (2): 35–36. doi:10.2344/0003-3006-60.2.35. PMC 3683877. PMID 23763556.
- Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, Paszat LF (February 2010). "Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study". BMJ. 340: c693. doi:10.1136/bmj.c693. PMC 2817754. PMID 20142325.
- ^ Isbister GK, Bowe SJ, Dawson A, Whyte IM (2004). "Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose". Journal of Toxicology. Clinical Toxicology. 42 (3): 277–285. doi:10.1081/CLT-120037428. PMID 15362595. S2CID 43121327.
- Borys DJ, Setzer SC, Ling LJ, Reisdorf JJ, Day LC, Krenzelok EP (1992). "Acute fluoxetine overdose: a report of 234 cases". The American Journal of Emergency Medicine. 10 (2): 115–120. doi:10.1016/0735-6757(92)90041-U. PMID 1586402.
- Oström M, Eriksson A, Thorson J, Spigset O (1996). "Fatal overdose with citalopram". Lancet. 348 (9023): 339–340. doi:10.1016/S0140-6736(05)64513-8. PMID 8709713. S2CID 5287418.
- Sporer KA (August 1995). "The serotonin syndrome. Implicated drugs, pathophysiology and management". Drug Safety. 13 (2): 94–104. doi:10.2165/00002018-199513020-00004. PMID 7576268. S2CID 19809259.
- Gupta R (2012). Veterinary Toxicology : Basic and Clinical Principles (2 ed.). Boston: Academic Press. pp. xii + 1438. ISBN 978-0-12-385926-6.
- ^ Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH (October 2010). Practice Guideline for the Treatment of Patients With Major Depressive Disorder (PDF) (third ed.). American Psychiatric Association. ISBN 978-0-89042-338-7. Archived (PDF) from the original on 2020-08-07. Retrieved 2018-07-22.
- Renoir T (2013). "Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved". Frontiers in Pharmacology. 4: 45. doi:10.3389/fphar.2013.00045. PMC 3627130. PMID 23596418.
- Goodman LS, Brunton LL, Chabner B, Knollmann BC (2001). Goodman and Gilman's pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 459–461. ISBN 978-0-07-162442-8.
- ^ Kolb, Bryan and Wishaw Ian. An Introduction to Brain and Behavior. New York: Worth Publishers 2006, Print.
- O'Brien FE, O'Connor RM, Clarke G, Dinan TG, Griffin BT, Cryan JF (October 2013). "P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents". Neuropsychopharmacology. 38 (11): 2209–2219. doi:10.1038/npp.2013.120. PMC 3773671. PMID 23670590.
- Shadfar S, Kim YG, Katila N, Neupane S, Ojha U, Bhurtel S, Srivastav S, Jeong GS, Park PH, Hong JT, Choi DY (January 2018). "Neuroprotective Effects of Antidepressants via Upregulation of Neurotrophic Factors in the MPTP Model of Parkinson's Disease". Molecular Neurobiology. 55 (1): 554–566. doi:10.1007/s12035-016-0342-0. PMID 27975170. S2CID 3322646.
- ^ Hindmarch I, Hashimoto K (April 2010). "Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered". Human Psychopharmacology. 25 (3): 193–200. doi:10.1002/hup.1106. PMID 20373470. S2CID 26491662.
- ^ Albayrak Y, Hashimoto K (2017). "Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders". Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Advances in Experimental Medicine and Biology. Vol. 964. pp. 153–161. doi:10.1007/978-3-319-50174-1_11. ISBN 978-3-319-50172-7. PMID 28315270.
- Kishimoto A, Todani A, Miura J, Kitagaki T, Hashimoto K (May 2010). "The opposite effects of fluvoxamine and sertraline in the treatment of psychotic major depression: a case report". Annals of General Psychiatry. 9: 23. doi:10.1186/1744-859X-9-23. PMC 2881105. PMID 20492642.
- Bafna SL, Patel DJ, Mehta JD (August 1972). "Separation of ascorbic acid and 2-keto-L-gulonic acid". Current Neuropharmacology. 61 (8): 1333–1334. doi:10.2174/1570159X14666151208113700. PMC 5050394. PMID 27640518.
- Köhler S, Cierpinsky K, Kronenberg G, Adli M (January 2016). "The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants". Journal of Psychopharmacology. 30 (1): 13–22. doi:10.1177/0269881115609072. PMID 26464458. S2CID 21501578.
- Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF (May 2018). "Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis". Molecular Neurobiology. 55 (5): 4195–4206. doi:10.1007/s12035-017-0632-1. PMID 28612257. S2CID 4040496. Archived from the original on 2020-08-07. Retrieved 2018-09-17.
- Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W, Mosiołek A, Waszkiewicz N, Szulc A (January 2018). "Effect of antidepressant treatment on peripheral inflammation markers – A meta-analysis". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 80 (Pt C): 217–226. doi:10.1016/j.pnpbp.2017.04.026. PMID 28445690. S2CID 34659323.
- Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, Smit JH, de Jonge P, Penninx BW (February 2012). "Association of depressive disorders, depression characteristics and antidepressant medication with inflammation". Translational Psychiatry. 2 (2): e79. doi:10.1038/tp.2012.8. PMC 3309556. PMID 22832816.
- ^ Kalkman HO, Feuerbach D (July 2016). "Antidepressant therapies inhibit inflammation and microglial M1-polarization". Pharmacology & Therapeutics. 163: 82–93. doi:10.1016/j.pharmthera.2016.04.001. PMID 27101921.
- ^ Nazimek K, Strobel S, Bryniarski P, Kozlowski M, Filipczak-Bryniarska I, Bryniarski K (June 2017). "The role of macrophages in anti-inflammatory activity of antidepressant drugs". Immunobiology. 222 (6): 823–830. doi:10.1016/j.imbio.2016.07.001. PMID 27453459.
- ^ Gobin V, Van Steendam K, Denys D, Deforce D (May 2014). "Selective serotonin reuptake inhibitors as a novel class of immunosuppressants". International Immunopharmacology. 20 (1): 148–156. doi:10.1016/j.intimp.2014.02.030. PMID 24613205.
- Saha M, Rizzo SA, Ramanathan M, Hightower RM, Santostefano KE, Terada N, Finkel RS, Berg JS, Chahin N, Pacak CA, Wagner RE, Alexander MS, Draper I, Kang PB (July 2019). "Selective serotonin reuptake inhibitors ameliorate MEGF10 myopathy". Human Molecular Genetics. 28 (14): 2365–2377. doi:10.1093/hmg/ddz064. PMC 6606856. PMID 31267131.
- Rasmussen-Torvik LJ, McAlpine DD (2007). "Genetic screening for SSRI drug response among those with major depression: great promise and unseen perils". Depression and Anxiety. 24 (5): 350–357. doi:10.1002/da.20251. PMID 17096399. S2CID 24257390.
- Anderson IM (April 2000). "Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability". Journal of Affective Disorders. 58 (1): 19–36. doi:10.1016/S0165-0327(99)00092-0. PMID 10760555.
- ^ Shelton RC (2009). "Serotonin norepinephrine reuptake inhibitors: similarities and differences". Primary Psychiatry. 16 (4): 25. Archived from the original on 2020-08-07. Retrieved 2017-08-26.
- Montgomery SA (July 2008). "Tolerability of serotonin norepinephrine reuptake inhibitor antidepressants". CNS Spectrums. 13 (7 Suppl 11): 27–33. doi:10.1017/s1092852900028297. PMID 18622372. S2CID 24692832.
- Waller DG, Sampson T (2017). Medical Pharmacology and Therapeutics E-Book. Elsevier Health Sciences. pp. 302–. ISBN 978-0-7020-7190-4.
- Kornstein SG, Clayton AH (2010). Women's Mental Health, An Issue of Psychiatric Clinics – E-Book. Elsevier Health Sciences. pp. 389–. ISBN 978-1-4557-0061-5. Archived from the original on 2023-01-14. Retrieved 2017-08-26.
- Bruno A, Morabito P, Spina E, Muscatello MR (2016). "The Role of Levomilnacipran in the Management of Major Depressive Disorder: A Comprehensive Review". Current Neuropharmacology. 14 (2): 191–199. doi:10.2174/1570159x14666151117122458. PMC 4825949. PMID 26572745.
- ^ Mandrioli R, Protti M, Mercolini L (2018). "New-Generation, non-SSRI Antidepressants: Therapeutic Drug Monitoring and Pharmacological Interactions. Part 1: SNRIs, SMSs, SARIs". Current Medicinal Chemistry. 24 (7): 772–792. doi:10.2174/0929867324666170712165042. PMID 28707591.
- ^ Ayd FJ (2000). Lexicon of Psychiatry, Neurology, and the Neurosciences. Lippincott Williams & Wilkins. pp. 581–. ISBN 978-0-7817-2468-5. Archived from the original on 2023-01-14. Retrieved 2017-08-26.
- ^ Progress in Drug Research. Birkhäuser. 2012. pp. 80–82. ISBN 978-3-0348-8391-7.
- ^ Moltzen EK, Bang-Andersen B (2006). "Serotonin reuptake inhibitors: the corner stone in treatment of depression for half a century – a medicinal chemistry survey". Current Topics in Medicinal Chemistry. 6 (17): 1801–1823. doi:10.2174/156802606778249810. PMID 17017959.
- ^ Haddad PM (1999). "Selective Serotonin Reuptake Inhibitors (SSRIs) Past, Present and Future. Edited by S. Clare Standford, R.G. Landes Company". Human Psychopharmacology: Clinical and Experimental. 15 (6). Austin, Texas: 471. doi:10.1002/1099-1077(200008)15:6<471::AID-HUP211>3.0.CO;2-4. ISBN 1-57059-649-2.
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (January 2008). "Selective publication of antidepressant trials and its influence on apparent efficacy". The New England Journal of Medicine. 358 (3): 252–260. CiteSeerX 10.1.1.486.455. doi:10.1056/NEJMsa065779. PMID 18199864.
- Ebrahim S, Bance S, Athale A, Malachowski C, Ioannidis JP (February 2016). "Meta-analyses with industry involvement are massively published and report no caveats for antidepressants". Journal of Clinical Epidemiology. 70: 155–163. doi:10.1016/j.jclinepi.2015.08.021. PMID 26399904.
- Healy D, Aldred G (June 2005). "Antidepressant drug use & the risk of suicide". International Review of Psychiatry. 17 (3): 163–172. CiteSeerX 10.1.1.482.5522. doi:10.1080/09540260500071624. PMID 16194787. S2CID 6599566.
- Lapierre YD (September 2003). "Suicidality with selective serotonin reuptake inhibitors: Valid claim?". Journal of Psychiatry & Neuroscience. 28 (5): 340–347. PMC 193980. PMID 14517577.
- Khan A, Khan S, Kolts R, Brown WA (April 2003). "Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports". The American Journal of Psychiatry. 160 (4): 790–792. doi:10.1176/appi.ajp.160.4.790. PMID 12668373. S2CID 20755149.
- Kaizar EE, Greenhouse JB, Seltman H, Kelleher K (2006). "Do antidepressants cause suicidality in children? A Bayesian meta-analysis". Clinical Trials. 3 (2): 73–90, discussion 91–8. doi:10.1191/1740774506cn139oa. PMID 16773951. S2CID 41954145.
- Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ (June 2012). "Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine". Archives of General Psychiatry. 69 (6): 580–587. doi:10.1001/archgenpsychiatry.2011.2048. PMC 3367101. PMID 22309973.
- Christou A, Papadavid G, Dalias P, Fotopoulos V, Michael C, Bayona JM, Piña B, Fatta-Kassinos D (March 2019). "Ranking of crop plants according to their potential to uptake and accumulate contaminants of emerging concern". Environmental Research. 170. Elsevier BV: 422–432. Bibcode:2019ER....170..422C. doi:10.1016/j.envres.2018.12.048. hdl:10261/202657. PMID 30623890. S2CID 58564142.
- Fitzgerald KT, Bronstein AC (February 2013). "Selective serotonin reuptake inhibitor exposure". Topics in Companion Animal Medicine. 28 (1): 13–17. doi:10.1053/j.tcam.2013.03.003. ISSN 1946-9837. PMID 23796482.
External links
| Anxiolytics (N05B) | |
|---|---|
| 5-HT1ARTooltip 5-HT1A receptor agonists | |
| GABAARTooltip GABAA receptor PAMsTooltip positive allosteric modulators |
|
| Hypnotics | |
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Antipsychotics | |
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| OCD pharmacotherapies | |
|---|---|
| Antidepressants |
|
| Others | |
| Monoamine reuptake inhibitors | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DATTooltip Dopamine transporter (DRIsTooltip Dopamine reuptake inhibitors) |
| ||||||||||||||
| NETTooltip Norepinephrine transporter (NRIsTooltip Norepinephrine reuptake inhibitors) |
| ||||||||||||||
| SERTTooltip Serotonin transporter (SRIsTooltip Serotonin reuptake inhibitors) |
| ||||||||||||||
| VMATsTooltip Vesicular monoamine transporters | |||||||||||||||
| Others |
| ||||||||||||||
| See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Sigma receptor modulators | |
|---|---|
| σ1 |
|
| σ2 |
|
| Unsorted |
|
| See also: Receptor/signaling modulators | |