| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Potassium selenide" – news · newspapers · books · scholar · JSTOR (March 2020) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
| IUPAC name Potassium selenide | |
| Other names Dipotassium selenide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.817 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | K2Se |
| Molar mass | 157.16 |
| Appearance | clearish wet crystal |
| Density | 2.29 g/cm |
| Melting point | 800 °C (1,470 °F; 1,070 K) |
| Solubility in water | reacts |
| Structure | |
| Crystal structure | cubic: antifluorite |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | toxic |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H301, H331, H373, H410 |
| Precautionary statements | P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P361, P363, P391, P403+P233, P405, P501 |
| Related compounds | |
| Other anions | Potassium oxide Potassium sulfide Potassium telluride Potassium polonide |
| Other cations | Lithium selenide Sodium selenide Rubidium selenide Caesium selenide |
| Related compounds | Potassium selenate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Potassium selenide (K2Se) is an inorganic compound formed from selenium and potassium.
Production
It can be produced by the reaction of selenium and potassium. If the two are combined in liquid ammonia, the purity is higher.
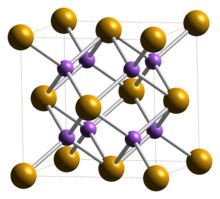
Crystal structure
Potassium selenide has a cubic, antifluorite crystal structure.
References
- Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 (, p. 692, at Google Books).
- Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 (, p. 336, at Google Books).
- "Potassium selenide" (2017) at ChemicalBook (database).
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |
| Salts and covalent derivatives of the selenide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |