| Part of a series on | |||||||
| Continuum mechanics | |||||||
|---|---|---|---|---|---|---|---|
| Fick's laws of diffusion | |||||||
Laws
|
|||||||
| Solid mechanics | |||||||
Fluid mechanics
|
|||||||
Rheology
|
|||||||
| Scientists | |||||||
Gay-Lussac's law usually refers to Joseph-Louis Gay-Lussac's law of combining volumes of gases, discovered in 1808 and published in 1809. However, it sometimes refers to the proportionality of the volume of a gas to its absolute temperature at constant pressure. The latter law was published by Gay-Lussac in 1802, but in the article in which he described his work, he cited earlier unpublished work from the 1780s by Jacques Charles. Consequently, the volume-temperature proportionality is usually known as Charles's Law.
Law of combining volumes
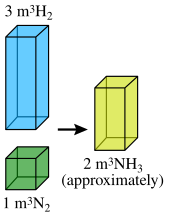
The law of combining volumes states that when gases chemically react together, they do so in amounts by volume which bear small whole-number ratios (the volumes calculated at the same temperature and pressure).
The ratio between the volumes of the reactant gases and the gaseous products can be expressed in simple whole numbers.
For example, Gay-Lussac found that two volumes of hydrogen react with one volume of oxygen to form two volumes of gaseous water. Expressed concretely, 100 mL of hydrogen combine with 50 mL of oxygen to give 100 mL of water vapor: Hydrogen(100 mL) + Oxygen(50 mL) = Water(100 mL). Thus, the volumes of hydrogen and oxygen which combine (i.e., 100mL and 50mL) bear a simple ratio of 2:1, as also is the case for the ratio of product water vapor to reactant oxygen.
Based on Gay-Lussac's results, Amedeo Avogadro hypothesized in 1811 that, at the same temperature and pressure, equal volumes of gases (of whatever kind) contain equal numbers of molecules (Avogadro's law). He pointed out that if this hypothesis is true, then the previously stated result
- 2 volumes of hydrogen + 1 volume of oxygen = 2 volume of gaseous water
could also be expressed as
- 2 molecules of hydrogen + 1 molecule of oxygen = 2 molecule of water.
The law of combining volumes of gases was announced publicly by Joseph Louis Gay-Lussac on the last day of 1808, and published in 1809. Since there was no direct evidence for Avogadro's molecular theory, very few chemists adopted Avogadro's hypothesis as generally valid until the Italian chemist Stanislao Cannizzaro argued convincingly for it during the First International Chemical Congress in 1860.
Pressure-temperature law
In the 17th century Guillaume Amontons discovered a regular relationship between the pressure and temperature of a gas at constant volume. Some introductory physics textbooks still define the pressure-temperature relationship as Gay-Lussac's law. Gay-Lussac primarily investigated the relationship between volume and temperature and published it in 1802, but his work did cover some comparison between pressure and temperature. Given the relative technology available to both men, Amontons could only work with air as a gas, whereas Gay-Lussac was able to experiment with multiple types of common gases, such as oxygen, nitrogen, and hydrogen.
Volume-temperature law
Regarding the volume-temperature relationship, Gay-Lussac attributed his findings to Jacques Charles because he used much of Charles's unpublished data from 1787 – hence, the law became known as Charles's law or the Law of Charles and Gay-Lussac.
Amontons's, Charles', and Boyle's law form the combined gas law. These three gas laws in combination with Avogadro's law can be generalized by the ideal gas law.
Gay-Lussac used the formula acquired from ΔV/V = αΔT to define the rate of expansion α for gases. For air, he found a relative expansion ΔV/V = 37.50% and obtained a value of α = 37.50%/100 °C = 1/266.66 °C which indicated that the value of absolute zero was approximately 266.66 °C below 0 °C. The value of the rate of expansion α is approximately the same for all gases and this is also sometimes referred to as Gay-Lussac's Law. See the introduction to this article, and Charles's Law.
See also
- Avogadro's law – Relationship between volume and amount of a gas at constant temperature and pressure
- Boyle's law – Relation between gas pressure and volume
- Charles's law – Relationship between volume and temperature of a gas at constant pressure
- Combined gas law – Combination of Charles', Boyle's and Gay-Lussac's gas laws
References
- "Sur la combinaison des substances gazeuses, les unes avec les autres," Mémoires de physique et de chimie de la Société d’Arcueil, vol. 2 (1809), 207-34.
- "Sur la dilatation des gaz," Annales de chimie, 43 (1802), 137-75.
- Gay-Lussac (1809) "Mémoire sur la combinaison des substances gazeuses, les unes avec les autres" (Memoir on the combination of gaseous substances with each other), Mémoires de la Société d'Arcueil 2: 207–234. Available in English at: Le Moyne College.
- "Joseph-Louis Gay-Lussac". chemistryexplained.com.
- Hartley Harold (1966). "Stanislao Cannizzaro, F.R.S. (1826–1910) and the First International Chemical Conference at Karlsruhe". Notes and Records of the Royal Society of London. 21 (1): 56–63. doi:10.1098/rsnr.1966.0006. S2CID 58453894.
- Tippens, Paul E. (2007). Physics, 7th ed. McGraw-Hill. 386–387.
- Cooper, Crystal (Feb. 11, 2010). "Gay-Lussac's Law". Bright Hub Engineering. Retrieved from http://www.brighthubengineering.com/hvac/26213-gay-lussacs-law/ on July 8, 2013.
- Verma, K.S. - Cengage Physical Chemistry Part 1 Archived 2021-05-06 at the Wayback Machine - Section 5.6.3
- Crosland, Maurice P. (2004). Gay-Lussac: Scientist and Bourgeois. Cambridge University Press. 119–120.
- Asimov, Isaac (1966). Understanding Physics – Motion, Sound, and Heat. Walker and Co. 191–192.
- Gay-Lussac (1802), "Recherches sur la dilatation des gaz et des vapeurs" (Researches on the expansion of gases and vapors), Annales de Chimie 43: 137–175. On page 157, Gay-Lussac mentions the unpublished findings of Charles: "Avant d'aller plus loin, je dois prévenir que quoique j'eusse reconnu un grand nombre de fois que les gaz oxigène, azote, hydrogène et acide carbonique, et l'air atmosphérique se dilatent également depuis 0° jusqu'a 80°, le cit. Charles avait remarqué depuis 15 ans la même propriété dans ces gaz; mais n'avant jamais publié ses résultats, c'est par le plus grand hasard que je les ai connus." (Before going further, I should inform that although I had recognized many times that the gases oxygen, nitrogen, hydrogen, and carbonic acid , and atmospheric air also expand from 0° to 80°, citizen Charles had noticed 15 years ago the same property in these gases; but having never published his results, it is by the merest chance that I knew of them.) Available in English at: Le Moyne College.
- Gay-Lussac (1802). "Recherches sur la dilatation des gaz et des vapeurs". Annales de chimie, ou, Recueil de mémoires concernant la chimie (in French).
Further reading
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry. Holt, Rinehart and Winston. ISBN 978-0-03-056537-3.
- Guch, Ian (2003). The Complete Idiot's Guide to Chemistry. Alpha, Penguin Group Inc. ISBN 978-1-59257-101-7.
- Mascetta, Joseph A. (1998). How to Prepare for the SAT II Chemistry. Barron's. ISBN 978-0-7641-0331-5.
| Mole concepts | |
|---|---|
| Constants | |
| Physical quantities | |
| Laws | |
