Enzyme-catalyzed proximity labeling (PL), also known as proximity-based labeling, is a laboratory technique that labels biomolecules, usually proteins or RNA, proximal to a protein of interest. By creating a gene fusion in a living cell between the protein of interest and an engineered labeling enzyme, biomolecules spatially proximal to the protein of interest can then be selectively marked with biotin for pulldown and analysis. Proximity labeling has been used for identifying the components of novel cellular structures and for determining protein-protein interaction partners, among other applications.
History
Before the development of proximity labeling, determination of protein proximity in cells relied on studying protein-protein interactions through methods such as affinity purification-mass spectrometry and proximity ligation assays.
DamID is a method developed in 2000 by Steven Henikoff for identifying parts of the genome proximal to a chromatin protein of interest. DamID relies on a DNA methyltransferase fusion to the chromatin protein to nonnaturally methylate DNA, which can then be subsequently sequenced to reveal genome methylation sites near the protein. Researchers were guided by the fusion protein strategy of DamID to create a method for site-specific labeling of protein targets, culminating in the creation of the biotin protein labelling-based BioID in 2012. Alice Ting and the Ting lab at Stanford University have engineered several proteins that demonstrate improvements in biotin-based proximity labeling efficacy and speed.
Principles
Proximity labeling relies on a labeling enzyme that can biotinylate nearby biomolecules promiscuously. Biotin labeling can be achieved through several different methods, depending on the species of labeling enzyme.
- BioID, also known as BirA*, is a mutant E. coli biotin ligase that catalyzes the activation of biotin by ATP. The activated biotin is short-lived and thus can only diffuse to a region proximal to BioID. Labeling is achieved when the activated biotin reacts with nearby amines, such as the lysine sidechain amines found in proteins. TurboID is a biotin ligase engineered via yeast surface display directed evolution. TurboID enables ~10 minute labeling times instead of the ~18 hour labeling times required by BioID.
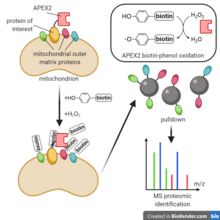
- APEX is an ascorbate peroxidase derivative reliant on hydrogen peroxide for catalyzing the oxidation of biotin-tyramide, also known as biotin-phenol, to a short-lived and reactive biotin-phenol free radical. Labeling is achieved when this intermediate reacts with various functional groups of nearby biomolecules. APEX can also be used for local deposition of diaminobenzidine, a precursor for an electron microscopy stain. APEX2 is a derivative of APEX engineered via yeast surface display directed evolution. APEX2 shows improved labeling efficiency and cellular expression levels.
To label proteins nearby a protein of interest, a typical proximity labeling experiment begins by cellular expression of an APEX2 fusion to the protein of interest, which localizes to the protein of interest's native environment. Cells are next incubated with biotin-phenol, then briefly with hydrogen peroxide, initiating biotin-phenol free radical generation and labeling. To minimize cellular damage, the reaction is then quenched using an antioxidant buffer. Cells are lysed and the labeled proteins are pulled down with streptavidin beads. The proteins are digested with trypsin, and finally the resulting peptidic fragments are analyzed using shotgun proteomics methods such as LC-MS/MS or SPS-MS.
If instead a protein fusion is not genetically accessible (such as in human tissue samples) but an antibody for the protein of interest is known, proximity labeling can still be enabled by fusing a labeling enzyme with the antibody, then incubating the fusion with the sample.
Applications
Proximity labeling methods have been used to study the proteomes of biological structures that are otherwise difficult to isolate purely and completely, such as cilia, mitochondria, postsynaptic clefts, p-bodies, stress granules, and lipid droplets.
Fusion of APEX2 with G-protein coupled receptors (GPCRs) allows for both tracking GPCR signaling at a 20-second temporal resolution and also identification of unknown GPCR-linked proteins.
Proximity labeling has also been used for transcriptomics and interactomics. In 2019, Alice Ting and the Ting lab have used APEX to identify RNA localized to specific cellular compartments. In 2019, BioID has been tethered to the beta-actin mRNA transcript to study its localization dynamics. Proximity labeling has also been used to find interaction partners of heterodimeric protein phosphatases, of the miRISC (microRNA-induced silencing complex) protein Ago2, and of ribonucleoproteins.
Recent developments
TurboID-based proximity labeling has been used to identify regulators of a receptor involved in the innate immune response, a NOD-like receptor. BioID-based proximity labeling has been used to identify the molecular composition of breast cancer cell invadopodia, which are important for metastasis. Biotin-based proximity labeling studies demonstrate increased protein tagging of intrinsically disordered regions, suggesting that biotin-based proximity labeling can be used to study the roles of IDRs. A photosensitizer nucleus-targeted small molecule has also been developed for photoactivatable proximity labeling.
Photocatalytic-based Proximity Labeling
A new frontier in the field of proximity labeling exploits the utility of photocatalysis to achieve high spatial and temporal resolution of proximal protein microenvironments. This photocatalytic technology leverages the photonic energy of iridium-based photocatalysts to activate diazirine probes that can tag proximal proteins within a tight radius of about four nanometers. This technology was developed by the Merck Exploratory Science Center in collaboration with researchers at Princeton University.
References
- ^ Roux, Kyle J.; Kim, Dae In; Raida, Manfred; Burke, Brian (2012-03-19). "A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells". Journal of Cell Biology. 196 (6): 801–810. doi:10.1083/jcb.201112098. ISSN 0021-9525. PMC 3308701. PMID 22412018.
- ^ Han, Shuo; Li, Jiefu; Ting, Alice Y (2018-06-01). "Proximity labeling: spatially resolved proteomic mapping for neurobiology". Current Opinion in Neurobiology. Neurotechnologies. 50: 17–23. doi:10.1016/j.conb.2017.10.015. ISSN 0959-4388. PMC 6726430. PMID 29125959.
- ^ Trinkle-Mulcahy, Laura (2019-01-31). "Recent advances in proximity-based labeling methods for interactome mapping". F1000Research. 8: 135. doi:10.12688/f1000research.16903.1. ISSN 2046-1402. PMC 6357996. PMID 30774936.
- Steensel, Bas van; Henikoff, Steven (April 2000). "Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase". Nature Biotechnology. 18 (4): 424–428. doi:10.1038/74487. ISSN 1546-1696. PMID 10748524. S2CID 30350384.
- ^ Branon, Tess C.; Bosch, Justin A.; Sanchez, Ariana D.; Udeshi, Namrata D.; Svinkina, Tanya; Carr, Steven A.; Feldman, Jessica L.; Perrimon, Norbert; Ting, Alice Y. (2018-10-01). "Efficient proximity labeling in living cells and organisms with TurboID". Nature Biotechnology. 36 (9): 880–887. doi:10.1038/nbt.4201. ISSN 1546-1696. PMC 6126969. PMID 30125270.
- ^ Rhee, Hyun-Woo; Zou, Peng; Udeshi, Namrata D.; Martell, Jeffrey D.; Mootha, Vamsi K.; Carr, Steven A.; Ting, Alice Y. (2013-03-15). "Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging". Science. 339 (6125): 1328–1331. Bibcode:2013Sci...339.1328R. doi:10.1126/science.1230593. ISSN 0036-8075. PMC 3916822. PMID 23371551.
- Lam, Stephanie S.; Martell, Jeffrey D.; Kamer, Kimberli J.; Deerinck, Thomas J.; Ellisman, Mark H.; Mootha, Vamsi K.; Ting, Alice Y. (January 2015). "Directed evolution of APEX2 for electron microscopy and proximity labeling". Nature Methods. 12 (1): 51–54. doi:10.1038/nmeth.3179. hdl:1721.1/110613. ISSN 1548-7105. PMC 4296904. PMID 25419960.
- ^ Kalocsay, Marian (2019). "APEX Peroxidase-Catalyzed Proximity Labeling and Multiplexed Quantitative Proteomics". In Sunbul, Murat; Jäschke, Andres (eds.). Proximity Labeling. Methods in Molecular Biology. Vol. 2008. Springer. pp. 41–55. doi:10.1007/978-1-4939-9537-0_4. ISBN 978-1-4939-9537-0. PMID 31124087. S2CID 163166385.
{{cite book}}:|work=ignored (help) - Rees, Johanna S.; Li, Xue-Wen; Perrett, Sarah; Lilley, Kathryn S.; Jackson, Antony P. (2015-11-01). "Protein Neighbors and Proximity Proteomics". Molecular & Cellular Proteomics. 14 (11): 2848–2856. doi:10.1074/mcp.R115.052902. ISSN 1535-9476. PMC 4638030. PMID 26355100.
- Bar, Daniel Z; Atkatsh, Kathleen; Tavarez, Urraca; Erdos, Michael R; Gruenbaum, Yosef; Collins, Francis S (February 2018). "Biotinylation by antibody recognition - A method for proximity labeling". Nature Methods. 15 (2): 127–133. doi:10.1038/nmeth.4533. ISSN 1548-7091. PMC 5790613. PMID 29256494.
- Mick, David U.; Rodrigues, Rachel B.; Leib, Ryan D.; Adams, Christopher M.; Chien, Allis S.; Gygi, Steven P.; Nachury, Maxence V. (2015-11-23). "Proteomics of Primary Cilia by Proximity Labeling". Developmental Cell. 35 (4): 497–512. doi:10.1016/j.devcel.2015.10.015. ISSN 1878-1551. PMC 4662609. PMID 26585297.
- Youn, Ji-Young; Dunham, Wade H.; Hong, Seo Jung; Knight, James D.R.; Bashkurov, Mikhail; Chen, Ginny I.; Bagci, Halil; Rathod, Bhavisha; MacLeod, Graham; Eng, Simon W.M.; Angers, Stéphane (February 2018). "High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies". Molecular Cell. 69 (3): 517–532.e11. doi:10.1016/j.molcel.2017.12.020. ISSN 1097-2765. PMID 29395067.
- Bersuker, Kirill; Peterson, Clark W. H.; To, Milton; Sahl, Steffen J.; Savikhin, Victoria; Grossman, Elizabeth A.; Nomura, Daniel K.; Olzmann, James A. (2018-01-08). "A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes". Developmental Cell. 44 (1): 97–112.e7. doi:10.1016/j.devcel.2017.11.020. ISSN 1534-5807. PMC 5764092. PMID 29275994.
- Paek, Jaeho; Kalocsay, Marian; Staus, Dean P.; Wingler, Laura; Pascolutti, Roberta; Paulo, Joao A.; Gygi, Steven P.; Kruse, Andrew C. (6 April 2017). "Multidimensional Tracking of GPCR Signaling via Peroxidase-Catalyzed Proximity Labeling". Cell. 169 (2): 338–349.e11. doi:10.1016/j.cell.2017.03.028. ISSN 1097-4172. PMC 5514552. PMID 28388415.
- Lobingier, Braden T.; Hüttenhain, Ruth; Eichel, Kelsie; Miller, Kenneth B.; Ting, Alice Y.; von Zastrow, Mark; Krogan, Nevan J. (6 April 2017). "An Approach to Spatiotemporally Resolve Protein Interaction Networks in Living Cells". Cell. 169 (2): 350–360.e12. doi:10.1016/j.cell.2017.03.022. ISSN 1097-4172. PMC 5616215. PMID 28388416.
- Shields, Emily J.; Petracovici, Ana F.; Bonasio, Roberto (2019-04-15). "lncRedibly versatile: biochemical and biological functions of long noncoding RNAs". Biochemical Journal. 476 (7): 1083–1104. doi:10.1042/BCJ20180440. ISSN 0264-6021. PMC 6745715. PMID 30971458.
- Fazal, Furqan M.; Han, Shuo; Parker, Kevin R.; Kaewsapsak, Pornchai; Xu, Jin; Boettiger, Alistair N.; Chang, Howard Y.; Ting, Alice Y. (2019-07-11). "Atlas of Subcellular RNA Localization Revealed by APEX-Seq". Cell. 178 (2): 473–490.e26. doi:10.1016/j.cell.2019.05.027. ISSN 0092-8674. PMC 6786773. PMID 31230715.
- Mukherjee, Joyita; Hermesh, Orit; Eliscovich, Carolina; Nalpas, Nicolas; Franz-Wachtel, Mirita; Maček, Boris; Jansen, Ralf-Peter (2019-06-25). "β-Actin mRNA interactome mapping by proximity biotinylation". Proceedings of the National Academy of Sciences. 116 (26): 12863–12872. Bibcode:2019PNAS..11612863M. doi:10.1073/pnas.1820737116. ISSN 0027-8424. PMC 6600913. PMID 31189591.
- Zhang, Yongliang; Song, Gaoyuan; Lal, Neeraj K.; Nagalakshmi, Ugrappa; Li, Yuanyuan; Zheng, Wenjie; Huang, Pin-jui; Branon, Tess C.; Ting, Alice Y.; Walley, Justin W.; Dinesh-Kumar, Savithramma P. (2019-07-19). "TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity". Nature Communications. 10 (1): 3252. Bibcode:2019NatCo..10.3252Z. doi:10.1038/s41467-019-11202-z. ISSN 2041-1723. PMC 6642208. PMID 31324801.
- Thuault, Sylvie; Mamelonet, Claire; Salameh, Joëlle; Ostacolo, Kevin; Chanez, Brice; Salaün, Danièle; Baudelet, Emilie; Audebert, Stéphane; Camoin, Luc; Badache, Ali (2020-04-22). "A proximity-labeling proteomic approach to investigate invadopodia molecular landscape in breast cancer cells". Scientific Reports. 10 (1): 6787. Bibcode:2020NatSR..10.6787T. doi:10.1038/s41598-020-63926-4. ISSN 2045-2322. PMC 7176661. PMID 32321993.
- Minde, David-Paul; Ramakrishna, Manasa; Lilley, Kathryn S. (2020-01-22). "Biotin proximity tagging favours unfolded proteins and enables the study of intrinsically disordered regions". Communications Biology. 3 (1): 38. doi:10.1038/s42003-020-0758-y. ISSN 2399-3642. PMC 6976632. PMID 31969649.
- Tamura, Tomonori; Takato, Mikiko; Shiono, Keiya; Hamachi, Itaru (2019-12-05). "Development of a Photoactivatable Proximity Labeling Method for the Identification of Nuclear Proteins". Chemistry Letters. 49 (2): 145–148. doi:10.1246/cl.190804. ISSN 0366-7022.
- Geri, Jacob; et al. 2020. "Microenvironment mapping via Dexter energy transfer on immune cells". Science.
- ^ Cross, Ryan. 2020. "Merck and Princeton scientists create method to map cell-surface microenvironments". Chemical And Engineering News.