| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Pschorr cyclization" – news · newspapers · books · scholar · JSTOR (December 2021) (Learn how and when to remove this message) |
| Pschorr cyclization | |||||
|---|---|---|---|---|---|
| Named after | Robert Pschorr | ||||
| Reaction type | Ring forming reaction | ||||
| Reaction | |||||
| |||||
| Identifiers | |||||
| Organic Chemistry Portal | pschorr-reaction | ||||
| Conditions | |||||
| Catalyst | copper | ||||
The Pschorr cyclization is a name reaction in organic chemistry, which was named after its discoverer, the German chemist Robert Pschorr (1868-1930). It describes the intramolecular substitution of aromatic compounds via aryldiazonium salts as intermediates and is catalyzed by copper. The reaction is a variant of the Gomberg-Bachmann reaction. The following reaction scheme shows the Pschorr cyclization for the example of phenanthrene:

Reaction mechanism
In the course of the Pschorr cyclization, a diazotization of the starting compound occurs, so that an aryldiazonium salt is formed as intermediate. For this, sodium nitrite is added to hydrochloric acid to obtain nitrous acid. The nitrous acid is protonated and reacts with another equivalent of nitrous acid to the intermediate 1 which is later used for the diazotization of the aromatic amine:

The intermediate 1 reacts in the following way with the starting compound:
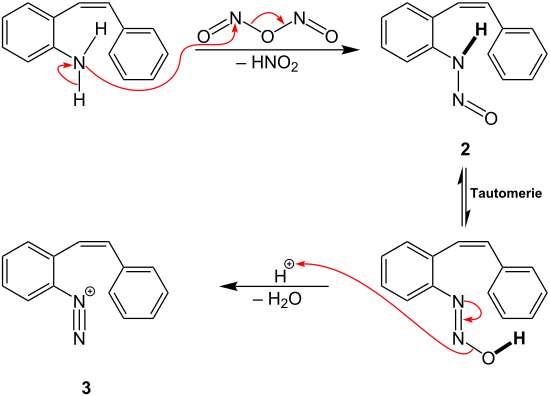
Intermediate 1 replaces a hydrogen atom from the amino group of the starting compound. A nitroso group is introduced as new substituent, producing under the release of nitrous acid intermediate 2. Intermediate 2 then reacts via a tautomerism and dehydration to the aryldiazonium cation 3.

Nitrogen is then cleaved from the aryldiazonium cation 3 by the use of the copper catalyst. The aryl radical thus formed reacts via ring closure to the intermediate stage 4. Finally, rearomatization takes place using again the copper catalyst and phenanthrene is formed.
Atom economy
The Pschorr cyclization has a relatively good atom economy, since essentially only nitrogen is produced as a waste material. For the diazotization, two equivalents of nitrous acid are used, of which one equivalent is being re-formed in the course of the reaction. The copper is used in catalytic amounts only and does therefore not affect the atomic efficiency of the reaction. However, when considering the atom economy it has to be mentioned that the Pschorr cyclization has often only low yields.
References
- Pschorr, Robert (1896), "Neue Synthese des Phenanthrens und seiner Derivate", Berichte der deutschen chemischen Gesellschaft (in German), vol. 29, no. 1, pp. 496–501, doi:10.1002/cber.18960290198
- ^ Cepanec, Ivica (2004), Synthesis of biaryls (1st ed.), Amsterdam: Elsevier, pp. 25–27, ISBN 9780080444123
- ^ Li, Jie Jack (2014), Name reactions: A collection of detailed mechanisms and synthetic applications (5th ed.), Cham: Springer, pp. 499–500, doi:10.1007/978-3-319-03979-4, ISBN 978-3-319-03979-4, S2CID 93616494