| This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (February 2023) |
| Orf2-like Prenyltransferase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
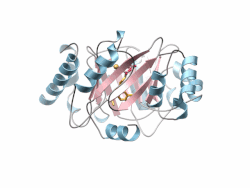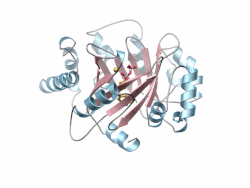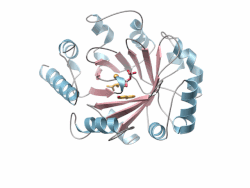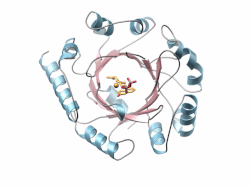 Crystal structure of a PT-barrel protein. Crystal structure of a PT-barrel protein. | |||||||||||
| Identifiers | |||||||||||
| Symbol | PTase_Orf2 | ||||||||||
| Pfam | PF11468 | ||||||||||
| InterPro | IPR020965 | ||||||||||
| SCOP2 | 1zb6 / SCOPe / SUPFAM | ||||||||||
| |||||||||||
The PT-barrel, is a novel protein fold that was discovered in the crystal structure of the prenyltransferase, Orf2 from Streptomyces sp. strain CL190.
Structure
The PT-barrel consists of a closed β-sheet comprising ten anti-parallel β-strands arranged around a central β-barrel core, itself surrounded by a ring of α-helices forming the outer, solvent exposed surface of the barrel.
The secondary connectivity nearly conforms to an (ααββ)5 classification, but is more specifically described using the (ααββ)4-(αββ)−α nomenclature, where helices 6 and 8, both involved in inter-protein contacts in the crystal lattice, display a helical “kink”. The most hydrophobic section of the PT-barrel is the region residing between the outer surface of the cylindrical β-barrel and the belt of surrounding α-helices. Additionally, a number of hydrophobic residues located inside the barrel accommodate the prenyl tail of the Geranyl-diPhosphate (GPP) and GSPP molecules, while the diphosphate or the thio-diphosphate head groups of substrate and substrate analogs, respectively, point toward the “upper”, more polar end of the barrel where a Mg ion is coordinated. Finally, the bottom of the barrel is capped by a short C-terminal helix (α11).
References
- Kuzuyama T, Noel JP, Richard SB (June 2005). "Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products". Nature. 435 (7044): 983–7. Bibcode:2005Natur.435..983K. doi:10.1038/nature03668. PMC 2874460. PMID 15959519.
- Koehl P (July 2005). "Relaxed specificity in aromatic prenyltransferases". Nat. Chem. Biol. 1 (2): 71–2. doi:10.1038/nchembio0705-71. PMID 16408001. S2CID 10831640.
- Botta B, Delle Monache G, Menendez P, Boffi A (December 2005). "Novel prenyltransferase enzymes as a tool for flavonoid prenylation". Trends Pharmacol. Sci. 26 (12): 606–8. doi:10.1016/j.tips.2005.09.012. PMID 16229901.
Press Releases
Promiscuous Catalytic Activity Possessed by Novel Enzyme Structure, June 15, 2005
SSRL Science Highlight July 2005
LightSource.org Press Release Number: PR-SSRL-05-3
| Protein tertiary structure | |
|---|---|
| General | |
| All-α folds: | |
| All-β folds: | |
| α/β folds: | |
| α+β folds: | |
| Irregular folds: | |