| |
| Names | |
|---|---|
| Preferred IUPAC name 1-ethan-1-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
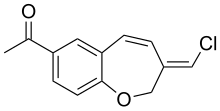
| Chemical formula | C13H11ClO2 |
| Molar mass | 234.678 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Pterulone is a fungal metabolite. It was initially isolated from the mycelium and liquid cultures of wood-decay fungus in the genus Pterula. The compound inhibits eukaryotic respiration by targeting the mitochondrial NADH:ubiquinone oxidoreductase.
References
- Engler M, Anke T, Sterner O, Brandt U (1997). "Pterulinic acid and pterulone, two novel inhibitors of NADH:ubiquinone oxidoreductase (complex I) produced by a Pterula species. I. Production, isolation and biological activities". Journal of Antibiotics. 50 (4): 325–9. doi:10.7164/antibiotics.50.325. PMID 9186558.
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |