| |

| |
| Names | |
|---|---|
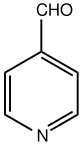
| Preferred IUPAC name Pyridine-4-carbaldehyde | |
| Other names 4-formylpyridine, 4-pyridinaldehyde, isonicotinaldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.666 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H5NO |
| Molar mass | 107.112 g·mol |
| Appearance | colorless liquid |
| Melting point | 4 °C (39 °F; 277 K) |
| Boiling point | 198 °C (388 °F; 471 K) |
| Acidity (pKa) | 4.72 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H317, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Pyridine-4-carbaldehyde is an organic compound with the formula C5H4NCHO. It is one of three isomeric pyridinaldehydes. The other isomers are pyridine-2-carboxaldehyde and pyridine-3-carboxaldehyde. Pyridine-4-carboxaldehyde is a colorless liquid, although aged samples can appear yellow or even brown. It undergoes many reactions expected for aromatic aldehydes such as reductive amination and Schiff base formation. It condenses with pyrrole to give tetrapyridylporphyrin. The pKa has been experimentally determined by NMR spectroscopy to be 4.72.
References
- Chougnet, Antoinette; Woggon, Wolf-D. (2013). "Enantioselective Nitroaldol (Henry) Reaction of p-Nitrobenzaldehyde and Nitromethane Using a Copper (II) Complex Derived from (R,R)-1,2-Diaminocyclohexane: (1S)-1-(4-Nitrophenyl)-2-nitroethane-1-ol". Organic Syntheses. 90: 52. doi:10.15227/orgsyn.090.0052.
- Drain, Charles Michael; Lehn, Jean-Marie (1994). "Self-Assembly of Square Multiporphyrin Arrays by Metal Ion Coordination". Journal of the Chemical Society, Chemical Communications (19): 2313. doi:10.1039/c39940002313.
- Handloser, Carolyn S.; Chakrabarty, M. R.; Mosher, Melvyn W. (July 1973). "Experimental determination of pKa values by use of NMR chemical shift". Journal of Chemical Education. 50 (7): 510. doi:10.1021/ed050p510. ISSN 0021-9584.