A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na[BO2] and borax (Na)2[B4O7]. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3 but they are alkoxides.
Natural occurrence
Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, where they make an important contribution to the absorption of low frequency sound in seawater.
Borates also occur in plants, including almost all fruits.
Anions
The main borate anions are:
- tetrahydroxyborate [B(OH)4], found in sodium tetrahydroxyborate Na[B(OH)4].
- orthoborate [BO3], found in trisodium orthoborate Na3[BO3]
, found in the calcium yttrium borosilicate oxyapatite Ca
3Y
7BO
4(SiO
4)
5O- perborate [B2O4(OH)4], as in sodium perborate Na2[H4B2O8]
- metaborate [BO2] or its cyclic trimer [B3O6], found in sodium metaborate Na3[B3O6]
- diborate [B2O5], found in magnesium diborate (suanite) Mg2[B2O5] ,
- triborate [B3O7], found in calcium aluminium triborate (johachidolite) Ca[AlB3O7] ,
- tetraborate [B4O7], found in anhydrous borax Na2[B4O7]
- tetrahydroxytetraborate [B4O5(OH)4], found in borax "decahydrate" Na2[B4O5(OH)4]·8H2O
- tetraborate(6-) [B4O9] found in lithium tetraborate(6-) Li6[B4O9]
- pentaborate [B5O8] or [B10O16], found in sodium pentaborate Na2[B10O16]·10H2O
- octaborate [B8O13] found in disodium octaborate Na2[B8O13]
-
 The structure of the tetrahydroxyborate ion ([B(OH)4]). This anion has a tetrahedral molecular geometry at the boron atom.
The structure of the tetrahydroxyborate ion ([B(OH)4]). This anion has a tetrahedral molecular geometry at the boron atom.
-
 The structure of the orthoborate ion ([BO3]). This anion has a trigonal planar molecular geometry.
The structure of the orthoborate ion ([BO3]). This anion has a trigonal planar molecular geometry.
-
 The structure of the trimer of the metaborate ion ([B3O6]). This anion is a cyclic molecule and has a trigonal planar molecular geometry at the boron atoms. All nine atoms of this anion lie on the same plane.
The structure of the trimer of the metaborate ion ([B3O6]). This anion is a cyclic molecule and has a trigonal planar molecular geometry at the boron atoms. All nine atoms of this anion lie on the same plane.
-
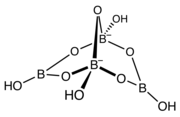 The structure of the tetrahydroxytetraborate ion ([B4O5(OH)4]). This anion is a bridged bicyclic molecule, contains oxygen atoms bridging the boron atoms, which are linked to four hydroxyl groups (−OH), one per each boron atom. The anion has a tetrahedral molecular geometry at the two tetracoordinated boron atoms, and has a trigonal planar molecular geometry at the two tricoordinated boron atoms.
The structure of the tetrahydroxytetraborate ion ([B4O5(OH)4]). This anion is a bridged bicyclic molecule, contains oxygen atoms bridging the boron atoms, which are linked to four hydroxyl groups (−OH), one per each boron atom. The anion has a tetrahedral molecular geometry at the two tetracoordinated boron atoms, and has a trigonal planar molecular geometry at the two tricoordinated boron atoms.
-
 The structure of the tetraborate ion ([B4O7]). This anion has the same topology as the tetrahydroxytetraborate ion, but without the hydroxyl groups and all boron atoms have a trigonal planar molecular geometry.
The structure of the tetraborate ion ([B4O7]). This anion has the same topology as the tetrahydroxytetraborate ion, but without the hydroxyl groups and all boron atoms have a trigonal planar molecular geometry.
-
 The structure of the perborate ion ([B2O4(OH)4]). This anion is a cyclic molecule and has a tetrahedral molecular geometry at the boron atoms. It contains two bridging peroxide groups (−O−O−) and four hydroxyl groups (−OH) attached to boron atoms, two per each boron. The ring has a chair conformation.
The structure of the perborate ion ([B2O4(OH)4]). This anion is a cyclic molecule and has a tetrahedral molecular geometry at the boron atoms. It contains two bridging peroxide groups (−O−O−) and four hydroxyl groups (−OH) attached to boron atoms, two per each boron. The ring has a chair conformation.
-
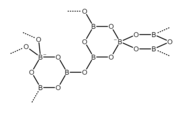 The structure of the repeating unit of the octaborate ion ([B8O13]) in the alpha form of disodium octaborate (α-Na2[B8O13]). This anion is cyclic and polymeric. It has a tetrahedral molecular geometry at the negatively charged boron atoms and a trigonal planar molecular geometry at the neutral boron atoms.
The structure of the repeating unit of the octaborate ion ([B8O13]) in the alpha form of disodium octaborate (α-Na2[B8O13]). This anion is cyclic and polymeric. It has a tetrahedral molecular geometry at the negatively charged boron atoms and a trigonal planar molecular geometry at the neutral boron atoms.
Preparation
In 1905, Burgess and Holt observed that fusing mixtures of boric oxide B2O3 and sodium carbonate Na2CO3 yielded on cooling two crystalline compounds with definite compositions, consistent with anhydrous borax Na2B4O7 (which can be written Na2O·2B2O3) and sodium octaborate Na2B8O13 (which can be written Na2O·4B2O3).
Structures
Borate anions (and functional groups) consist of trigonal planar BO3 and/or tetrahedral BO4 structural units, joined together via shared oxygen atoms (corners) or atom pairs (edges) into larger clusters so as to construct various ions such as [B2O5], [B3O8], [B4O12], [B5O6(OH)5], [B6O13], etc. These anions may be cyclic or linear in structure, and can further polymerize into infinite chains, layers, and tridimensional frameworks. The terminal (unshared) oxygen atoms in the borate anions may be capped with hydrogen atoms (−OH) or may carry a negative charge (−O).
The planar BO3 units may be stacked in the crystal lattice so as to have π-conjugated molecular orbitals, which often results in useful optical properties such as strong harmonics generation, birefringence, and UV transmission.
Polymeric borate anions may have linear chains of 2, 3 or 4 trigonal BO3 structural units, each sharing oxygen atoms with adjacent unit(s). as in LiBO2, contain chains of trigonal BO3 structural units. Other anions contain cycles; for instance, NaBO2 and KBO2 contain the cyclic [B3O6] ion, consisting of a six-membered ring of alternating boron and oxygen atoms with one extra oxygen atom attached to each boron atom.
The thermal expansion of crystalline borates is dominated by the fact that BO3 and BO4 polyhedra and rigid groups consisting of these polyhedra practically do not change their configuration and size upon heating, but sometimes rotate like hinges, which results in greatly anisotropic thermal expansion including linear negative expansion.
Reactions
Aqueous solution
In aqueous solution, boric acid B(OH)3 can act as a weak Brønsted acid, that is, a proton donor, with pKa ~ 9. However, it more often acts as a Lewis acid, accepting an electron pair from a hydroxide ion produced by the water autoprotolysis:
- B(OH)3 + 2 H2O ⇌ [B(OH)4] + H3O (pK = 8.98)
This reaction is very fast, with characteristic time less than 10 μs. Polymeric boron oxoanions are formed in aqueous solution of boric acid at pH 7–10 if the boron concentration is higher than about 0.025 mol/L. The best known of these is the tetraborate ion [B4O7], found in the mineral borax:
Other anions observed in solution are triborate(1−) and pentaborate(1−), in equilibrium with boric acid and tetrahydroxyborate according to the following overall reactions:
- 2B(OH)3 + [B(OH)4] ⇌ [B3O3(OH)4] + 3H2O (fast, pK = —1.92)
- 4B(OH)3 + [B(OH)4] ⇌ [B5O6(OH)4] + 6H2O (slow, pK = —2.05)
In the pH range 6.8 to 8.0, any alkali salts of "boric oxide" anions with general formula [BxOy(OH)z]((q) where 3x+q = 2y + z will eventually equilibrate in solution to a mixture of B(OH)3, [B(OH)4], [B3O3(OH)4], and [B5O6(OH)4].
These ions, similarly to the complexed borates mentioned above, are more acidic than boric acid itself. As a result of this, the pH of a concentrated polyborate solution will increase more than expected when diluted with water.
Borate salts
A number of metal borates are known. They can be obtained by treating boric acid or boron oxides with metal oxides.
Mixed anion salts
Some chemicals contain another anion in addition to borate. These include borate chlorides, borate carbonates, borate nitrates, borate sulfates, borate phosphates.
Complex oxyanions containing boron
More complex anions can be formed by condensing borate triangles or tetrahedra with other oxyanions to yield materials such as borosulfates, boroselenates, borotellurates, boroantimonates, borophosphates, or boroselenites.
Borosilicate glass, also known as pyrex, can be viewed as a silicate in which some units are replaced by centers, together with additional cations to compensate for the difference in valence states of Si(IV) and B(III). Because this substitution leads to imperfections, the material is slow to crystallise and forms a glass with low coefficient of thermal expansion, thus resistant to cracking when heated, unlike soda glass.
Uses

Common borate salts include sodium metaborate (NaBO2) and borax. Borax is soluble in water, so mineral deposits only occur in places with very low rainfall. Extensive deposits were found in Death Valley and shipped with twenty-mule teams from 1883 to 1889. In 1925, deposits were found at Boron, California on the edge of the Mojave Desert. The Atacama Desert in Chile also contains mineable borate concentrations.
Lithium metaborate, lithium tetraborate, or a mixture of both, can be used in borate fusion sample preparation of various samples for analysis by XRF, AAS, ICP-OES and ICP-MS. Borate fusion and energy dispersive X-ray fluorescence spectrometry with polarized excitation have been used in the analysis of contaminated soils.
Disodium octaborate tetrahydrate Na2B8O13·4H2O (commonly abbreviated DOT) is used as a wood preservative or fungicide. Zinc borate is used as a flame retardant.
Some borates with large anions and multiple cations, like K2Al2B2O7 and Cs3Zn6B9O21 have been considered for applications in nonlinear optics.
Borate esters
Borate esters are organic compounds, which are conveniently prepared by the stoichiometric condensation reaction of boric acid with alcohols (or their chalcogen analogs).
Thin films
Metal borate thin films have been grown by a variety of techniques, including liquid-phase epitaxy (e.g. FeBO3, β-BaB2O4), electron-beam evaporation (e.g. CrBO3, β-BaB2O4), pulsed laser deposition (e.g. β-BaB2O4, Eu(BO2)3), and atomic layer deposition (ALD). Growth by ALD was achieved using precursors composed of the tris(pyrazolyl)borate ligand and either ozone or water as the oxidant to deposit CaB2O4, SrB2O4, BaB2O4, Mn3(BO3)2, and CoB2O4 films.
Physiology
Borate anions are largely in the form of the undissociated acid in aqueous solution at physiological pH. No further metabolism occurs in either animals or plants. In animals, boric acid/borate salts are essentially completely absorbed following oral ingestion. Absorption occurs via inhalation, although quantitative data are unavailable. Limited data indicate that boric acid/salts are not absorbed through intact skin to any significant extent, although absorption occurs through skin that is severely abraded. It distributes throughout the body and is not retained in tissues, except for bone, and is rapidly excreted in the urine.
See also
- Nanochannel glass materials
- Porous glass
- Vycor glass
- Silly Putty
- Slime (toy)
- Tris(2,2,2-trifluoroethyl) borate
References
- "Underlying Physics and Mechanisms for the Absorption of Sound in Seawater". National Physical Laboratory. Retrieved 2008-04-21.
- Allen, A. H.; Tankard, A. R. (1904). "The Determination of Boric Acid in Cider, Fruits, etc". Analyst. 29 (October): 301–304. Bibcode:1904Ana....29..301A. doi:10.1039/an9042900301.
- "Tetraborate".
- Carrondo, M. A. A. F. de C. T.; Skapski, A. C. (1978). "Refinement of the X-ray crystal structure of the industrial bleaching agent disodium tetrahydroxo-di-μ-peroxo-diborate hexahydrate, Na2·6H2O". Acta Crystallogr B. 34: 3551. doi:10.1107/S0567740878011565.
- Bubnova, R. S.; Shepelev, Ju. F.; Sennova, N. A.; Filatov, S. K. (2002). "Thermal behaviour of the rigid boron-oxygen groups in the α-Na2B8O13 crystal structure". Zeitschrift für Kristallographie - Crystalline Materials. 217 (9): 444–450. Bibcode:2002ZK....217..444B. doi:10.1524/zkri.217.9.444.22881. S2CID 95388918.
- Charles Hutchens Burgess and Alfred Holt (1905): "Some physical characters of the sodium borates, with a new and rapid method for the determination of melting points." Proceedings of the Royal Society of London, volume 74, pages 285–295.doi:10.1098/rspl.1904.0112
- ^ Wiberg E. and Holleman A.F. (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Miriding Mutailipu, Min Zhang, Xiaoyu Dong, Yanna Chen, and Shilie Pan (2016): "Effects of the Orientation of Fundamental Building Blocks on Layered Structures Based on the Pentaborates". Inorganic Chemistry, volume 55, issue 20, pages 10608–10616. doi:10.1021/acs.inorgch
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 205. ISBN 978-0-08-037941-8.
- Rimma S. Bubnova and Stanislav K. Filatov (2008): "Strong anisotropic thermal expansion in borates". Basic Solid State Physics, volume 245, issue 11, pages 2469-2476. doi:10.1002/pssb.200880253
- Atkins; et al. (2010). Inorganic Chemistry (5th ed.). Oxford University Press. p. 334. ISBN 9780199236176.
- Ingri, N. (1962). "Equilibrium Studies of Polyanions. 8. On the First Equilibrium Steps in the Hydrolysis of Boric Acid, a Comparison between Equilibria in 0.1 M and 3.0 M NaClO4". Acta Chemica Scandinavica. 16 (2): 439–448. doi:10.3891/acta.chem.scand.16-0439. ISSN 0904-213X.
- ^ Robert K. Momii and Norman H. Nachtrieb (1967): "Nuclear Magnetic Resonance Study of Borate-Polyborate Equilibria in Aqueous Solution". Inorganic Chemistry, volume 6, issue 6, pages 1189-1192. doi:10.1021/ic50052a026
- Hettipathirana, Terrance D. (2004). "Simultaneous determination of parts-per-million level Cr, As, Cd and Pb, and major elements in low level contaminated soils using borate fusion and energy dispersive X-ray fluorescence spectrometry with polarized excitation". Spectrochimica Acta Part B: Atomic Spectroscopy. 59 (2): 223–229. Bibcode:2004AcSpB..59..223H. doi:10.1016/j.sab.2003.12.013.
- "Esters". The IUPAC Compendium of Chemical Terminology. 2014. doi:10.1351/goldbook.E02219.
- Yagupov, S.; Strugatsky, M.; Seleznyova, K.; Mogilenec, Yu.; Milyukova, E.; Maksimova, E.; Nauhatsky, I.; Drovosekov, A.; Kreines, N. (November 2016). "Iron borate films: Synthesis and characterization" (PDF). Journal of Magnetism and Magnetic Materials. 417: 338–343. Bibcode:2016JMMM..417..338Y. doi:10.1016/j.jmmm.2016.05.098.
- Liu, Junfang; He, Xiaoming; Xia, Changtai; Zhou, Guoqing; Zhou, Shengming; Xu, Jun; Yao, Wu; Qian, Liejia (July 2006). "Preparation of crystalline beta barium borate thin films on Sr-doped alpha barium borate substrates by liquid phase epitaxy". Thin Solid Films. 510 (1–2): 251–254. Bibcode:2006TSF...510..251L. doi:10.1016/j.tsf.2005.12.205.
- Jha, Menaka; Kshirsagar, Sachin D.; Ghanashyam Krishna, M.; Ganguli, Ashok K. (June 2011). "Growth and optical properties of chromium borate thin films". Solid State Sciences. 13 (6): 1334–1338. Bibcode:2011SSSci..13.1334J. doi:10.1016/j.solidstatesciences.2011.04.002.
- Maia, L. J. Q.; Feitosa, C. A. C.; De Vicente, F. S.; Mastelaro, V. R.; Li, M. Siu; Hernandes, A. C. (September 2004). "Structural and optical characterization of beta barium borate thin films grown by electron beam evaporation". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 22 (5): 2163–2167. Bibcode:2004JVSTA..22.2163M. doi:10.1116/1.1778409. ISSN 0734-2101.
- Xiao, R.-F.; Ng, L. C.; Yu, P.; Wong, G. K. L. (1995-07-17). "Preparation of crystalline beta barium borate (β-BaB2O4) thin films by pulsed laser deposition". Applied Physics Letters. 67 (3): 305–307. Bibcode:1995ApPhL..67..305X. doi:10.1063/1.115426. ISSN 0003-6951.
- Aleksandrovsky, A. S.; Krylov, A. S.; Potseluyko, A. M.; Seredkin, V. A.; Zaitsev, A. I.; Zamkov, A. V. (2006-02-09). Konov, Vitaly I.; Panchenko, Vladislav Y.; Sugioka, Koji; Veiko, Vadim P. (eds.). "Pulsed laser deposition of europium borate glass films and their optical and magneto-optical properties". Society of Photo-Optical Instrumentation Engineers (Spie) Conference Series. SPIE Proceedings. 6161: 61610A–61610A–7. Bibcode:2006SPIE.6161E..0AA. doi:10.1117/12.675020. S2CID 136530746.
- Saly, Mark J.; Munnik, Frans; Winter, Charles H. (2010). "Atomic layer deposition of CaB2O4 films using bis(tris(pyrazolyl)borate)calcium as a highly thermally stable boron and calcium source". Journal of Materials Chemistry. 20 (44): 9995. doi:10.1039/c0jm02280b. ISSN 0959-9428.
- Saly, Mark J.; Munnik, Frans; Winter, Charles H. (June 2011). "The Atomic Layer Deposition of SrB2O4 Films Using the Thermally Stable Precursor Bis(tris(pyrazolyl)borate)strontium". Chemical Vapor Deposition. 17 (4–6): 128–134. doi:10.1002/cvde.201006890.
- Saly, Mark J.; Munnik, Frans; Baird, Ronald J.; Winter, Charles H. (2009-08-25). "Atomic Layer Deposition Growth of BaB2O4 Thin Films from an Exceptionally Thermally Stable Tris(pyrazolyl)borate-Based Precursor". Chemistry of Materials. 21 (16): 3742–3744. doi:10.1021/cm902030d. ISSN 0897-4756. S2CID 93114230.
- ^ Klesko, Joseph P.; Bellow, James A.; Saly, Mark J.; Winter, Charles H.; Julin, Jaakko; Sajavaara, Timo (September 2016). "Unusual stoichiometry control in the atomic layer deposition of manganese borate films from manganese bis(tris(pyrazolyl)borate) and ozone". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 34 (5): 051515. Bibcode:2016JVSTA..34e1515K. doi:10.1116/1.4961385. ISSN 0734-2101.
- U.S. Environmental Protection Agency (2005), "Boric Acid/Sodium Borate Salts". HED Chapter of the Tolerance Reassessment Eligibility Decision Document (TRED), EPA-HQ-OPP-2005-0062-0004, p.11 (January 2006). As cited by PubChem.
External links
- Suanite at webmineral
- Johachidolite at webmineral
- Non-CCA Wood Preservatives: Guide to Selected Resources - National Pesticide Information Center Archived 2007-10-31 at the Wayback Machine
| Borates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||