A quantum heat engine is a device that generates power from the heat flow between hot and cold reservoirs. The operation mechanism of the engine can be described by the laws of quantum mechanics. The first realization of a quantum heat engine was pointed out by Scovil and Schulz-DuBois in 1959, showing the connection of efficiency of the Carnot engine and the 3-level maser. Quantum refrigerators share the structure of quantum heat engines with the purpose of pumping heat from a cold to a hot bath consuming power first suggested by Geusic, Schulz-DuBois, De Grasse and Scovil. When the power is supplied by a laser the process is termed optical pumping or laser cooling, suggested by Wineland and Hänsch. Surprisingly heat engines and refrigerators can operate up to the scale of a single particle thus justifying the need for a quantum theory termed quantum thermodynamics.
The 3-level amplifier as a quantum heat engine
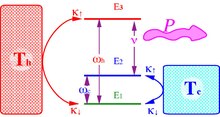
The three-level-amplifier is the template of a quantum device. It operates by employing a hot and cold bath to maintain population inversion between two energy levels which is used to amplify light by stimulated emission The ground state level (1-g) and the excited level (3-h) are coupled to a hot bath of temperature . The energy gap is . When the population on the levels equilibrate
where is the Planck constant and is the Boltzmann constant. The cold bath of temperature couples the ground (1-g) to an intermediate level (2-c) with energy gap . When levels 2-c and 1-g equilibrate then
- .
The device operates as an amplifier when levels (3-h) and (2-c) are coupled to an external field of frequency . For optimal resonance conditions . The efficiency of the amplifier in converting heat to power is the ratio of work output to heat input:
- .
Amplification of the field is possible only for positive gain (population inversion) . This is equivalent to . Inserting this expression into the efficiency formula leads to:
where is the Carnot cycle efficiency. Equality is obtained under a zero gain condition . The relation between the quantum amplifier and the Carnot efficiency was first pointed out by Scovil and Schultz-DuBois:
Reversing the operation driving heat from the cold bath to the hot bath by consuming power constitutes a refrigerator. The efficiency of the refrigerator defined as the coefficient of performance (COP) for the reversed device is:
Types
Quantum devices can operate either continuously or by a reciprocating cycle. Continuous devices include solar cells converting solar radiation to electrical power, thermoelectric where the output is current and lasers where the output power is coherent light. The primary example of a continuous refrigerator is optical pumping and laser cooling. Similarly to classical reciprocating engines, quantum heat engines also have a cycle that is divided into different strokes. A stroke is time segment in which a certain operation takes place (e.g. thermalization, or work extraction). Two adjacent strokes do not commute with each other. The most common reciprocating heat machines are the four-stroke machine, and the two-stroke machine. Reciprocating devices have been suggested operating either by the Carnot cycle or the Otto cycle.
In both types the quantum description allows to obtain equation of motion for the working medium and the heat flow from the reservoirs.
Quantum reciprocating heat engine and refrigerator
See also: Reciprocating heat engineQuantum versions of most of the common thermodynamic cycles have been studied, for example the Carnot cycle, Stirling cycle and Otto cycle.
The Otto cycle can serve as a template for other reciprocating cycles.

It is composed of the following four segments:
- Segment isomagnetic or isochoric process, partial equilibration with the cold bath under constant Hamiltonian. The dynamics of the working medium is characterized by the propagator .
- Segment magnetization or adiabatic compression, the external field changes expanding the gap between energy levels of the Hamiltonian. The dynamics is characterized by the propagator .
- Segment isomagnetic, or isochoric process partial equilibration with the hot bath described by the propagator .
- Segment demagnetization or adiabatic expansion reducing the energy gaps in the Hamiltonian, characterized by the propagator .
The propagator of the four stroke cycle becomes , which is the ordered product of the segment propagators:
The propagators are linear operators defined on a vector space which completely determines the state of the working medium. Common to all thermodynamic cycles the consecutive segment propagators do not commute . Commuting propagators will lead to zero power.
In a reciprocating quantum heat engine the working medium is a quantum system such as spin systems or an harmonic oscillator. For maximum power the cycle time should be optimized. There are two basic timescales in the reciprocating refrigerator the cycle time and the internal timescale . In general when the engine operates in quasi-adiabatic conditions. The only quantum effect can be found at low temperatures where the unit of energy of the device becomes instead of . The efficiency at this limit is , always smaller than the Carnot efficiency . At high temperature and for the harmonic working medium the efficiency at maximum power becomes which is the endoreversible thermodynamics result.
For shorter cycle times the working medium cannot follow adiabatically the change in the external parameter. This leads to friction-like phenomena. Extra power is required to drive the system faster. The signature of such dynamics is the development of coherence causing extra dissipation. Surprisingly the dynamics leading to friction is quantized meaning that frictionless solutions to the adiabatic expansion/compression can be found in finite time. As a result, optimization has to be carried out only with respect to the time allocated to heat transport. In this regime the quantum feature of coherence degrades the performance. Optimal frictionless performance is obtained when the coherence can be cancelled.
The shortest cycle times , sometimes termed sudden cycles, have universal features. In this case coherence contributes to the cycles power.
A two-stroke engine quantum cycle equivalent to the Otto cycle based on two qubits has been proposed. The first qubit has frequency and the second . The cycle is composed of a first stroke of partial equilibration of the two qubits with the hot and cold bath in parallel. The second power stroke is composed of a partial or full swap between the qubits. The swap operation is generated by a unitary transformation which preserves the entropy as a result it is a pure power stroke.
The quantum Otto cycle refrigerators shares the same cycle with magnetic refrigeration.
Continuous quantum engines
Continuous quantum engines are the quantum analogues of turbines. The work output mechanism is coupling to an external periodic field, typically the electromagnetic field. Thus the heat engine is a model for a laser. The models differ by the choice of their working substance and heat source and sink. Externally driven two-level, three level four-level and coupled harmonic oscillators have been studied.
The periodic driving splits the energy level structure of the working medium. This splitting allows the two level engine to couple selectively to the hot and cold baths and produce power. On the other hand, ignoring this splitting in the derivation of the equation of motion will violate the second law of thermodynamics.
Non thermal fuels have been considered for quantum heat engines. The idea is to increase the energy content of the hot bath without increasing its entropy. This can be achieved by employing coherence or a squeezed thermal bath. These devices do not violate the second law of thermodynamics.
Equivalence of reciprocating and continuous heat machines in the quantum regime
Two-stroke, Four-stroke, and continuous machine are very different from each other. However it was shown that there is a quantum regime where all these machines become thermodynamically equivalent to each other. While the intra cycle dynamics in the equivalence regime is very different in different engine types, when the cycle is completed they all turn out to provide the same amount of work and consume the same amount of heat (hence they share the same efficiency as well). This equivalence is associated with a coherent work extraction mechanism and has no classical analogue. These quantum features have been demonstrated experimentally.
Heat engines and open quantum systems
The elementary example operates under quasi equilibrium conditions. Its main quantum feature is the discrete energy level structure. More realistic devices operate out of equilibrium possessing friction heat leaks and finite heat flow. Quantum thermodynamics supplies a dynamical theory required for systems out of equilibrium such as heat engines, thus, inserting dynamics into thermodynamics. The theory of open quantum systems constitutes the basic theory. For heat engines a reduced description of the dynamics of the working substance is sought, tracing out the hot and cold baths. The starting point is the general Hamiltonian of the combined systems:
and the system Hamiltonian is time dependent. A reduced description leads to the equation of motion of the system:
where is the density operator describing the state of the working medium and is the generator of dissipative dynamics which includes the heat transport terms from the baths. Using this construction, the total change in energy of the sub-system becomes:
leading to the dynamical version of the first law of thermodynamics:
- The power
- Heat currents and .
The rate of entropy production becomes:
The global structure of quantum mechanics is reflected in the derivation of the reduced description. A derivation which is consistent with the laws of thermodynamics is based on the weak coupling limit. A thermodynamical idealization assumes that the system and the baths are uncorrelated, meaning that the total state of the combined system becomes a tensor product at all times:
Under these conditions the dynamical equations of motion become: where is the Liouville superoperator described in terms of the system's Hilbert space, where the reservoirs are described implicitly. Within the formalism of quantum open system, can take the form of the Gorini-Kossakowski-Sudarshan-Lindblad (GKS-L) Markovian generator or also known just as Lindblad equation . Theories beyond the weak coupling regime have been proposed.
The quantum absorption refrigerator
The absorption refrigerator is of unique importance in setting an autonomous quantum device. Such a device requires no external power and operates without external intervention in scheduling the operations . The basic construct includes three baths; a power bath, a hot bath and a cold bath. The tricycle model is the template for the absorption refrigerator.
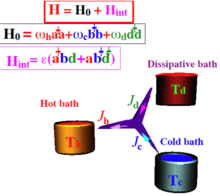
The tricycle engine has a generic structure. The basic model consists of three thermal baths: A hot bath with temperature , a cold bath with temperature and a work bath with temperature .
Each bath is connected to the engine via a frequency filter which can be modeled by three oscillators:
where , and are the filter frequencies on resonance .
The device operates as a refrigerator by removing an excitation from the cold bath as well as from the work bath and generating an excitation in the hot bath. The term in the Hamiltonian is non linear and crucial for an engine or a refrigerator.
where is the coupling strength.
The first-law of thermodynamics represents the energy balance of heat currents originating from the three baths and collimating on the system:
At steady state no heat is accumulated in the tricycle, thus . In addition, in steady state the entropy is only generated in the baths, leading to the second law of thermodynamics:
This version of the second-law is a generalisation of the statement of Clausius theorem; heat does not flow spontaneously from cold to hot bodies. When the temperature , no entropy is generated in the power bath. An energy current with no accompanying entropy production is equivalent to generating pure power: , where is the power output.
Quantum refrigerators and the third law of thermodynamics
There are seemingly two independent formulations of the third law of thermodynamics both originally were stated by Walther Nernst. The first formulation is known as the Nernst heat theorem, and can be phrased as:
- The entropy of any pure substance in thermodynamic equilibrium approaches zero as the temperature approaches zero.
The second formulation is dynamical, known as the unattainability principle:
- It is impossible by any procedure, no matter how idealized, to reduce any assembly to absolute zero temperature in a finite number of operations.
At steady state the second law of thermodynamics implies that the total entropy production is non-negative. When the cold bath approaches the absolute zero temperature, it is necessary to eliminate the entropy production divergence at the cold side when , therefore
For the fulfillment of the second law depends on the entropy production of the other baths, which should compensate for the negative entropy production of the cold bath. The first formulation of the third law modifies this restriction. Instead of the third law imposes , guaranteeing that at absolute zero the entropy production at the cold bath is zero: . This requirement leads to the scaling condition of the heat current .
The second formulation, known as the unattainability principle can be rephrased as;
- No refrigerator can cool a system to absolute zero temperature at finite time.
The dynamics of the cooling process is governed by the equation
where is the heat capacity of the bath. Taking and with , we can quantify this formulation by evaluating the characteristic exponent of the cooling process,
This equation introduce the relation between the characteristic exponents and . When then the bath is cooled to zero temperature in a finite time, which implies a violation of the third law. It is apparent from the last equation, that the unattainability principle is more restrictive than the Nernst heat theorem.
References
- ^ Scovil, H. E. D.; Schulz-DuBois, E. O. (1959). "Three-Level Masers as Heat Engines". Physical Review Letters. 2 (6): 262–263. Bibcode:1959PhRvL...2..262S. doi:10.1103/PhysRevLett.2.262. ISSN 0031-9007.
- Geusic, J. E.; Bois, E. O. Schulz-Du; De Grasse, R. W.; Scovil, H. E. D. (1959). "Three Level Spin Refrigeration and Maser Action at 1500 mc/sec". Journal of Applied Physics. 30 (7): 1113–1114. Bibcode:1959JAP....30.1113G. doi:10.1063/1.1776991. ISSN 0021-8979.
- D. J. Wineland and H. Dehmelt, Bull. Am. Phys. Soc. 20, 637 (1975)
- Hänsch, T.W.; Schawlow, A.L. (1975). "Cooling of gases by laser radiation". Optics Communications. 13 (1): 68–69. Bibcode:1975OptCo..13...68H. doi:10.1016/0030-4018(75)90159-5. ISSN 0030-4018.
- Letokhov, V.S.; Minogin, V.G.; Pavlik, B.D. (1976). "Cooling and trapping of atoms and molecules by a resonant laser field". Optics Communications. 19 (1): 72–75. Bibcode:1976OptCo..19...72L. doi:10.1016/0030-4018(76)90388-6. ISSN 0030-4018.
- ^ Alicki, R (1979). "The quantum open system as a model of the heat engine". Journal of Physics A: Mathematical and General. 12 (5): L103–L107. Bibcode:1979JPhA...12L.103A. doi:10.1088/0305-4470/12/5/007. ISSN 0305-4470.
- Yariv, Amnon (1989). Quantum Electronics, 3rd ed., Wiley. ISBN 0-471-60997-8
- Narevicius, Edvardas; Bannerman, S Travis; Raizen, Mark G (2009). "Single-photon molecular cooling". New Journal of Physics. 11 (5): 055046. arXiv:0808.1383. Bibcode:2009NJPh...11e5046N. doi:10.1088/1367-2630/11/5/055046. ISSN 1367-2630.
- ^ Kosloff, Ronnie; Levy, Amikam (2014). "Quantum Heat Engines and Refrigerators: Continuous Devices". Annual Review of Physical Chemistry. 65 (1): 365–393. arXiv:1310.0683. Bibcode:2014ARPC...65..365K. doi:10.1146/annurev-physchem-040513-103724. ISSN 0066-426X. PMID 24689798. S2CID 25266545.
- ^ Geva, Eitan; Kosloff, Ronnie (1992). "A quantum-mechanical heat engine operating in finite time. A model consisting of spin-1/2 systems as the working fluid". The Journal of Chemical Physics. 96 (4): 3054–3067. Bibcode:1992JChPh..96.3054G. doi:10.1063/1.461951. ISSN 0021-9606.
- ^ Bender, Carl M; Brody, Dorje C; Meister, Bernhard K (2000). "Quantum mechanical Carnot engine". Journal of Physics A: Mathematical and General. 33 (24): 4427–4436. arXiv:quant-ph/0007002. Bibcode:2000JPhA...33.4427B. doi:10.1088/0305-4470/33/24/302. ISSN 0305-4470. S2CID 5335.
- ^ Feldmann, Tova; Kosloff, Ronnie (2000). "Performance of discrete heat engines and heat pumps in finite time". Physical Review E. 61 (5): 4774–4790. arXiv:physics/0003007. Bibcode:2000PhRvE..61.4774F. doi:10.1103/PhysRevE.61.4774. ISSN 1063-651X. PMID 11031518. S2CID 2277942.
- Quan, H. T.; Liu, Yu-xi; Sun, C. P.; Nori, Franco (2007). "Quantum thermodynamic cycles and quantum heat engines". Physical Review E. 76 (3): 031105. arXiv:quant-ph/0611275. Bibcode:2007PhRvE..76c1105Q. doi:10.1103/PhysRevE.76.031105. ISSN 1539-3755. PMID 17930197. S2CID 3009953.
- Wu, F.; Chen, L.; Sun, F.; Wu, C.; Zhu, Yonghong (1998). "Performance and optimization criteria for forward and reverse quantum Stirling cycles". Energy Conversion and Management. 39 (8): 733–739. Bibcode:1998ECM....39..733W. doi:10.1016/S0196-8904(97)10037-1. ISSN 0196-8904.
- Kieu, T. D. (2006). "Quantum heat engines, the second law and Maxwell's daemon". The European Physical Journal D. 39 (1): 115–128. arXiv:quant-ph/0311157. Bibcode:2006EPJD...39..115K. doi:10.1140/epjd/e2006-00075-5. ISSN 1434-6060. S2CID 119382163.
- Feldmann, Tova; Kosloff, Ronnie (2003). "Quantum four-stroke heat engine: Thermodynamic observables in a model with intrinsic friction". Physical Review E. 68 (1): 016101. arXiv:quant-ph/0303046. Bibcode:2003PhRvE..68a6101F. doi:10.1103/PhysRevE.68.016101. ISSN 1063-651X. PMID 12935194. S2CID 23777311.
- ^ Rezek, Yair; Kosloff, Ronnie (2006). "Irreversible performance of a quantum harmonic heat engine". New Journal of Physics. 8 (5): 83. arXiv:quant-ph/0601006. Bibcode:2006NJPh....8...83R. doi:10.1088/1367-2630/8/5/083. ISSN 1367-2630.
- Campo, A. del; Goold, J.; Paternostro, M. (2014). "More bang for your buck: Super-adiabatic quantum engines". Scientific Reports. 4 (1): 6208. Bibcode:2014NatSR...4E6208C. doi:10.1038/srep06208. ISSN 2045-2322. PMC 4147366. PMID 25163421.
- Beau, Mathieu; Jaramillo, Juan; del Campo, Adolfo (2016). "Scaling-Up Quantum Heat Engines Efficiently via Shortcuts to Adiabaticity". Entropy. 18 (5): 168. arXiv:1603.06019. Bibcode:2016Entrp..18..168B. doi:10.3390/e18050168. ISSN 1099-4300.
- Feldmann, Tova; Kosloff, Ronnie (2012). "Short time cycles of purely quantum refrigerators". Physical Review E. 85 (5): 051114. arXiv:1204.4059. Bibcode:2012PhRvE..85e1114F. doi:10.1103/PhysRevE.85.051114. ISSN 1539-3755. PMID 23004710. S2CID 31174895.
- Allahverdyan, Armen E.; Hovhannisyan, Karen; Mahler, Guenter (2010). "Optimal refrigerator". Physical Review E. 81 (5): 051129. arXiv:1007.4307. Bibcode:2010PhRvE..81e1129A. doi:10.1103/PhysRevE.81.051129. ISSN 1539-3755. PMID 20866207. S2CID 12750223.
- Uzdin, Raam; Kosloff, Ronnie (2014). "The multilevel four-stroke swap engine and its environment". New Journal of Physics. 16 (9): 095003. arXiv:1404.6182. Bibcode:2014NJPh...16i5003U. doi:10.1088/1367-2630/16/9/095003. ISSN 1367-2630.
- Shirron, Peter J.; McCammon, Dan (2014). "Salt pill design and fabrication for adiabatic demagnetization refrigerators". Cryogenics. 62: 163–171. Bibcode:2014Cryo...62..163S. doi:10.1016/j.cryogenics.2014.03.022. ISSN 0011-2275.
- Gelbwaser-Klimovsky, D.; Alicki, R.; Kurizki, G. (2013). "Minimal universal quantum heat machine". Physical Review E. 87 (1): 012140. arXiv:1209.1190. Bibcode:2013PhRvE..87a2140G. doi:10.1103/PhysRevE.87.012140. ISSN 1539-3755. PMID 23410316. S2CID 18826566.
- Geva, Eitan; Kosloff, Ronnie (1996). "The quantum heat engine and heat pump: An irreversible thermodynamic analysis of the three-level amplifier". The Journal of Chemical Physics. 104 (19): 7681–7699. Bibcode:1996JChPh.104.7681G. doi:10.1063/1.471453. ISSN 0021-9606.
- Scully, M. O.; Chapin, K. R.; Dorfman, K. E.; Kim, M. B.; Svidzinsky, A. (2011). "Quantum heat engine power can be increased by noise-induced coherence". Proceedings of the National Academy of Sciences. 108 (37): 15097–15100. Bibcode:2011PNAS..10815097S. doi:10.1073/pnas.1110234108. ISSN 0027-8424. PMC 3174605. PMID 21876187.
- Harbola, Upendra; Rahav, Saar; Mukamel, Shaul (2012). "Quantum heat engines: A thermodynamic analysis of power and efficiency". EPL (Europhysics Letters). 99 (5): 50005. Bibcode:2012EL.....9950005H. doi:10.1209/0295-5075/99/50005. ISSN 0295-5075. S2CID 13833767.
- Kosloff, Ronnie (1984). "A quantum mechanical open system as a model of a heat engine". The Journal of Chemical Physics. 80 (4): 1625–1631. Bibcode:1984JChPh..80.1625K. doi:10.1063/1.446862. ISSN 0021-9606.
- Szczygielski, Krzysztof; Gelbwaser-Klimovsky, David; Alicki, Robert (2013). "Markovian master equation and thermodynamics of a two-level system in a strong laser field". Physical Review E. 87 (1): 012120. arXiv:1211.5665. Bibcode:2013PhRvE..87a2120S. doi:10.1103/PhysRevE.87.012120. ISSN 1539-3755. PMID 23410296. S2CID 25511420.
- Scully, M. O. (2003). "Extracting Work from a Single Heat Bath via Vanishing Quantum Coherence". Science. 299 (5608): 862–864. Bibcode:2003Sci...299..862S. doi:10.1126/science.1078955. ISSN 0036-8075. PMID 12511655. S2CID 120884236.
- Roßnagel, J.; Abah, O.; Schmidt-Kaler, F.; Singer, K.; Lutz, E. (2014). "Nanoscale Heat Engine Beyond the Carnot Limit". Physical Review Letters. 112 (3): 030602. arXiv:1308.5935. Bibcode:2014PhRvL.112c0602R. doi:10.1103/PhysRevLett.112.030602. ISSN 0031-9007. PMID 24484127. S2CID 1826585.
- Uzdin, Raam; Levy, Amikam; Kosloff, Ronnie (2015). "Equivalence of Quantum Heat Machines, and Quantum-Thermodynamic Signatures". Physical Review X. 5 (3): 031044. arXiv:1502.06592. Bibcode:2015PhRvX...5c1044U. doi:10.1103/PhysRevX.5.031044. ISSN 2160-3308.
- Klatzow, James; Becker, Jonas N; Ledingham, Patrick M; Weinzetl, Christian; Kaczmarek, Krzysztof T; Saunders, Dylan J; Nunn, Joshua; Walmsley, Ian A; Uzdin, Raam; Poem, Eilon (2019). "Experimental demonstration of quantum effects in the operation of microscopic heat engines". Physical Review Letters. 122 (11): 110601. arXiv:1710.08716. Bibcode:2019PhRvL.122k0601K. doi:10.1103/PhysRevLett.122.110601. ISSN 1079-7114. PMID 30951320. S2CID 206318713.
- Kosloff, Ronnie (2013). "Quantum Thermodynamics: A Dynamical Viewpoint". Entropy. 15 (12): 2100–2128. arXiv:1305.2268. Bibcode:2013Entrp..15.2100K. doi:10.3390/e15062100. ISSN 1099-4300.
- Ludovico, M. F.; Lim, J. S.; Moskalets, M.; Arrachea, L.; Sánchez, D. (21 April 2014). "Dynamical energy transfer in ac-driven quantum systems". Physical Review B. 89 (16): 161306. Bibcode:2014PhRvB..89p1306L. doi:10.1103/PhysRevB.89.161306. hdl:10261/116187. S2CID 119265583.
- Gallego, R; Riera, A; Eisert, J (2014). "Thermal machines beyond the weak coupling regime". New Journal of Physics. 16 (12): 125009. arXiv:1310.8349. Bibcode:2014NJPh...16l5009G. doi:10.1088/1367-2630/16/12/125009. ISSN 1367-2630.
- Esposito, Massimiliano; Ochoa, Maicol A.; Galperin, Michael (2015). "Quantum Thermodynamics: A Nonequilibrium Green's Function Approach". Physical Review Letters. 114 (8): 080602. arXiv:1411.1800. Bibcode:2015PhRvL.114h0602E. doi:10.1103/PhysRevLett.114.080602. ISSN 0031-9007. PMID 25768745. S2CID 11498686.
- Palao, José P.; Kosloff, Ronnie; Gordon, Jeffrey M. (2001). "Quantum thermodynamic cooling cycle". Physical Review E. 64 (5): 056130. arXiv:quant-ph/0106048. Bibcode:2001PhRvE..64e6130P. doi:10.1103/PhysRevE.64.056130. ISSN 1063-651X. PMID 11736037. S2CID 8201978.
- Linden, Noah; Popescu, Sandu; Skrzypczyk, Paul (2010). "How Small Can Thermal Machines Be? The Smallest Possible Refrigerator". Physical Review Letters. 105 (13): 130401. arXiv:0908.2076. Bibcode:2010PhRvL.105m0401L. doi:10.1103/PhysRevLett.105.130401. ISSN 0031-9007. PMID 21230755. S2CID 2707740.
- Levy, Amikam; Kosloff, Ronnie (2012). "Quantum Absorption Refrigerator". Physical Review Letters. 108 (7): 070604. arXiv:1109.0728. Bibcode:2012PhRvL.108g0604L. doi:10.1103/PhysRevLett.108.070604. ISSN 0031-9007. PMID 22401189. S2CID 6981288.
- Landsberg, P. T. (1956). "Foundations of Thermodynamics". Reviews of Modern Physics. 28 (4): 363–392. Bibcode:1956RvMP...28..363L. doi:10.1103/RevModPhys.28.363. ISSN 0034-6861.
- Levy, Amikam; Alicki, Robert; Kosloff, Ronnie (2012). "Quantum refrigerators and the third law of thermodynamics". Physical Review E. 85 (6): 061126. arXiv:1205.1347. Bibcode:2012PhRvE..85f1126L. doi:10.1103/PhysRevE.85.061126. ISSN 1539-3755. PMID 23005070. S2CID 24251763.
Further reading
Deffner, Sebastian and Campbell, Steve. "Quantum Thermodynamics: An introduction to the thermodynamics of quantum information", (Morgan & Claypool Publishers, 2019).
F. Binder, L. A. Correa, C. Gogolin, J. Anders, G. Adesso (eds.) "Thermodynamics in the Quantum Regime. Fundamental Aspects and New Directions." (Springer 2018)
Gemmer, Jochen, M. Michel, and Günter Mahler. "Quantum thermodynamics. Emergence of thermodynamic behavior within composite quantum systems. 2." (2009).
Petruccione, Francesco, and Heinz-Peter Breuer. The theory of open quantum systems. Oxford university press, 2002.
External links
- Deffner, Sebastian (2019). Quantum Thermodynamics. doi:10.1088/2053-2571/ab21c6. ISBN 978-1-64327-658-8. S2CID 195791624.
 .
The energy gap is
.
The energy gap is  . When the population on the levels equilibrate
. When the population on the levels equilibrate

 is the
is the  is the
is the  couples the ground (1-g) to an intermediate level (2-c)
with energy gap
couples the ground (1-g) to an intermediate level (2-c)
with energy gap  .
When levels 2-c and 1-g equilibrate then
.
When levels 2-c and 1-g equilibrate then
 .
. .
For optimal resonance conditions
.
For optimal resonance conditions  .
The efficiency of the amplifier in converting heat to power is the ratio of work output to heat input:
.
The efficiency of the amplifier in converting heat to power is the ratio of work output to heat input:
 .
. .
This is equivalent to
.
This is equivalent to  .
Inserting this expression into the efficiency formula leads to:
.
Inserting this expression into the efficiency formula leads to:

 is the
is the  .
The relation between the quantum amplifier and the
.
The relation between the quantum amplifier and the 
 plane where the energy entropy and the
plane where the energy entropy and the  isomagnetic or
isomagnetic or  .
.
 .
. isomagnetic, or isochoric process partial equilibration with the hot bath described by the propagator
isomagnetic, or isochoric process partial equilibration with the hot bath described by the propagator  .
.
 .
. , which is the ordered product of the segment propagators:
, which is the ordered product of the segment propagators:

 .
Commuting propagators will lead to zero power.
.
Commuting propagators will lead to zero power.
 and the internal
timescale
and the internal
timescale  . In general when
. In general when  the
engine operates in quasi-adiabatic conditions. The only quantum effect can be found at low temperatures
where the unit of energy of the device becomes
the
engine operates in quasi-adiabatic conditions. The only quantum effect can be found at low temperatures
where the unit of energy of the device becomes  instead of
instead of  .
The efficiency at this limit is
.
The efficiency at this limit is  , always smaller than the
, always smaller than the  which is the
which is the  , sometimes termed sudden cycles, have universal features. In this case coherence contributes to the cycles power.
, sometimes termed sudden cycles, have universal features. In this case coherence contributes to the cycles power.
 and the second
and the second  . The cycle is composed
of a first stroke of partial equilibration of the two qubits with the hot and cold bath in parallel.
The second power stroke is composed of a partial or full swap between the qubits.
The swap operation is generated by a unitary transformation which preserves the
. The cycle is composed
of a first stroke of partial equilibration of the two qubits with the hot and cold bath in parallel.
The second power stroke is composed of a partial or full swap between the qubits.
The swap operation is generated by a unitary transformation which preserves the 
 is time dependent.
A reduced description leads to the equation of motion of the system:
is time dependent.
A reduced description leads to the equation of motion of the system:

 is the density operator describing the state of the working medium and
is the density operator describing the state of the working medium and  is the generator of dissipative dynamics
which includes the heat transport terms from the baths.
Using this construction, the total change in energy of the sub-system becomes:
is the generator of dissipative dynamics
which includes the heat transport terms from the baths.
Using this construction, the total change in energy of the sub-system becomes:


 and
and  .
.

 where
where  is the Liouville superoperator described in terms of the system's Hilbert space,
where the reservoirs are described implicitly.
Within the formalism of quantum open system,
is the Liouville superoperator described in terms of the system's Hilbert space,
where the reservoirs are described implicitly.
Within the formalism of quantum open system,  can take the form of the
Gorini-Kossakowski-Sudarshan-Lindblad (GKS-L)
can take the form of the
Gorini-Kossakowski-Sudarshan-Lindblad (GKS-L)  . Heat flows from the power reservoir and cold bath to the hot bath.
. Heat flows from the power reservoir and cold bath to the hot bath. .
.

 are the filter frequencies on resonance
are the filter frequencies on resonance  .
.
 in the Hamiltonian is non linear
and crucial for an engine or a refrigerator.
in the Hamiltonian is non linear
and crucial for an engine or a refrigerator.

 is the coupling strength.
is the coupling strength.

 .
In addition, in steady state the entropy is only generated in the baths, leading to the
.
In addition, in steady state the entropy is only generated in the baths, leading to the 
 , no entropy is generated in the power bath.
An energy current with no accompanying
, no entropy is generated in the power bath.
An energy current with no accompanying  , where
, where  is the power output.
is the power output.
 , therefore
, therefore

 the fulfillment of the
the fulfillment of the  the third law imposes
the third law imposes  ,
guaranteeing that at absolute zero the entropy production at the cold bath is zero:
,
guaranteeing that at absolute zero the entropy production at the cold bath is zero:  .
This requirement leads to the scaling condition of the heat current
.
This requirement leads to the scaling condition of the heat current  .
.

 is the heat capacity of the bath. Taking
is the heat capacity of the bath. Taking  with
with  , we can quantify this formulation by evaluating the characteristic exponent
, we can quantify this formulation by evaluating the characteristic exponent  of the cooling process,
of the cooling process,

 . When
. When  then the bath is cooled to zero temperature in a finite time, which implies a violation of the third law. It is apparent from the last equation, that the unattainability principle is more restrictive than the
then the bath is cooled to zero temperature in a finite time, which implies a violation of the third law. It is apparent from the last equation, that the unattainability principle is more restrictive than the