| |
| Clinical data | |
|---|---|
| Other names | RG-7795; ANA773; ANA-773 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
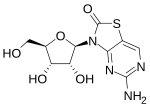
| Formula | C12H14N4O5S |
| Molar mass | 326.33 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
RG7795 (previously ANA773) is an antiviral drug candidate that as of 2015 had been in Phase II trials in hepatitis B. It is an orally-available prodrug of isatoribine, that was under development by Anadys Pharmaceuticals when it was acquired by Roche in 2011. Its active metabolite is an agonist of TLR7; activation of TLR7 causes secretion of endogenous type 1 interferons, which have antiviral activity.
As of 2021, development of RG7795 appears to be discontinued.
References
- "RG 7795". AdisInsight. Springer Nature Switzerland AG. Retrieved 28 August 2017.
- ^ Funk E, Kottilil S, Gilliam B, Talwani R (May 2014). "Tickling the TLR7 to cure viral hepatitis". Journal of Translational Medicine. 12: 129. doi:10.1186/1479-5876-12-129. PMC 4039542. PMID 24884741.
- "Inovio Goes It Alone on Hepatitis B Immunotherapy Vaccine as Roche Ends Collaboration". Genetic Engineering News. August 3, 2016.
- Sachin Bhagchandani, Jeremiah A.Johnson, and Darrell J.Irvine (2021). "Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants". Advanced Drug Delivery Reviews. 175: 113803. doi:10.1016/j.addr.2021.05.013. PMC 9003539. PMID 34058283.
{{cite journal}}: CS1 maint: multiple names: authors list (link)