Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of gold: due to relativistic effects, it is not silvery like most other metals.
The term relativistic effects was developed in light of the history of quantum mechanics. Initially, quantum mechanics was developed without considering the theory of relativity. Relativistic effects are those discrepancies between values calculated by models that consider relativity and those that do not. Relativistic effects are important for heavier elements with high atomic numbers, such as lanthanides and actinides.
Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation. These corrections affect the electrons differently depending on the electron speed compared with the speed of light. Relativistic effects are more prominent in heavy elements because only in these elements do electrons attain sufficient speeds for the elements to have properties that differ from what non-relativistic chemistry predicts.
History
Beginning in 1935, Bertha Swirles described a relativistic treatment of a many-electron system, despite Paul Dirac's 1929 assertion that the only imperfections remaining in quantum mechanics "give rise to difficulties only when high-speed particles are involved and are therefore of no importance in the consideration of the atomic and molecular structure and ordinary chemical reactions in which it is, indeed, usually sufficiently accurate if one neglects relativity variation of mass and velocity and assumes only Coulomb forces between the various electrons and atomic nuclei".
Theoretical chemists by and large agreed with Dirac's sentiment until the 1970s, when relativistic effects were observed in heavy elements. The Schrödinger equation had been developed without considering relativity in Schrödinger's 1926 article. Relativistic corrections were made to the Schrödinger equation (see Klein–Gordon equation) to describe the fine structure of atomic spectra, but this development and others did not immediately trickle into the chemical community. Since atomic spectral lines were largely in the realm of physics and not in that of chemistry, most chemists were unfamiliar with relativistic quantum mechanics, and their attention was on lighter elements typical for the organic chemistry focus of the time.
Dirac's opinion on the role relativistic quantum mechanics would play for chemical systems has been largely dismissed for two main reasons. First, electrons in s and p atomic orbitals travel at a significant fraction of the speed of light. Second, relativistic effects give rise to indirect consequences that are especially evident for d and f atomic orbitals.
Qualitative treatment
| This section needs additional citations for verification. Please help improve this article by adding citations to reliable sources in this section. Unsourced material may be challenged and removed. Find sources: "Relativistic quantum chemistry" – news · newspapers · books · scholar · JSTOR (August 2018) (Learn how and when to remove this message) |
| This article needs to be updated. Please help update this article to reflect recent events or newly available information. (December 2020) |
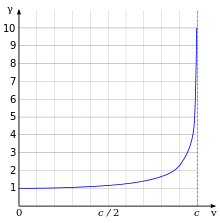
One of the most important and familiar results of relativity is that the relativistic mass of the electron increases as
where are the electron rest mass, velocity of the electron, and speed of light respectively. The figure at the right illustrates this relativistic effect as a function of velocity.
This has an immediate implication on the Bohr radius (), which is given by
where is the reduced Planck constant, and α is the fine-structure constant (a relativistic correction for the Bohr model).
Bohr calculated that a 1s orbital electron of a hydrogen atom orbiting at the Bohr radius of 0.0529 nm travels at nearly 1/137 the speed of light. One can extend this to a larger element with an atomic number Z by using the expression for a 1s electron, where v is its radial velocity, i.e., its instantaneous speed tangent to the radius of the atom. For gold with Z = 79, v ≈ 0.58c, so the 1s electron will be moving at 58% of the speed of light. Substituting this in for v/c in the equation for the relativistic mass, one finds that mrel = 1.22me, and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.
If one substitutes the "relativistic mass" into the equation for the Bohr radius it can be written

It follows that
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreases with increasing velocity.
When the Bohr treatment is extended to hydrogenic atoms, the Bohr radius becomes where is the principal quantum number, and Z is an integer for the atomic number. In the Bohr model, the angular momentum is given as . Substituting into the equation above and solving for gives
From this point, atomic units can be used to simplify the expression into;
Substituting this into the expression for the Bohr ratio mentioned above gives
At this point one can see that a low value of and a high value of results in . This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, and as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
Periodic table deviations
Mercury
Mercury (Hg) is a liquid down to approximately −39 °C, its melting point. Bonding forces are weaker for Hg–Hg bonds than for their immediate neighbors such as cadmium (m.p. 321 °C) and gold (m.p. 1064 °C). The lanthanide contraction only partially accounts for this anomaly. Because the 6s orbital is contracted by relativistic effects and may therefore only weakly contribute to any chemical bonding, Hg–Hg bonding must be mostly the result of van der Waals forces.
Mercury gas is mostly monatomic, Hg(g). Hg2(g) rarely forms and has a low dissociation energy, as expected due to the lack of strong bonds.
Au2(g) and Hg(g) are analogous with H2(g) and He(g) with regard to having the same nature of difference. The relativistic contraction of the 6s orbital leads to gaseous mercury sometimes being referred to as a pseudo noble gas.
Color of gold and caesium
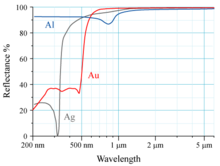

The reflectivity of aluminium (Al), silver (Ag), and gold (Au) is shown in the graph to the right. The human eye sees electromagnetic radiation with a wavelength near 600 nm as yellow. Gold absorbs blue light more than it absorbs other visible wavelengths of light; the reflected light reaching the eye is therefore lacking in blue compared with the incident light. Since yellow is complementary to blue, this makes a piece of gold under white light appear yellow to human eyes.
The electronic transition from the 5d orbital to the 6s orbital is responsible for this absorption. An analogous transition occurs in silver, but the relativistic effects are smaller than in gold. While silver's 4d orbital experiences some relativistic expansion and the 5s orbital contraction, the 4d–5s distance in silver is much greater than the 5d–6s distance in gold. The relativistic effects increase the 5d orbital's distance from the atom's nucleus and decrease the 6s orbital's distance. Due to the decreased 6s orbital distance, the electronic transition primarily absorbs in the violet/blue region of the visible spectrum, as opposed to the UV region.
Caesium, the heaviest of the alkali metals that can be collected in quantities sufficient for viewing, has a golden hue, whereas the other alkali metals are silver-white. However, relativistic effects are not very significant at Z = 55 for caesium (not far from Z = 47 for silver). The golden color of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended. For lithium through rubidium, this frequency is in the ultraviolet, but for caesium it reaches the blue-violet end of the visible spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium. Thus caesium transmits and partially absorbs violet light preferentially, while other colors (having lower frequency) are reflected; hence it appears yellowish.
Lead–acid battery
Without relativity, lead (Z = 82) would be expected to behave much like tin (Z = 50), so tin–acid batteries should work just as well as the lead–acid batteries commonly used in cars. However, calculations show that about 10 V of the 12 V produced by a 6-cell lead–acid battery arises purely from relativistic effects, explaining why tin–acid batteries do not work.
Inert-pair effect
Main article: Inert-pair effectIn Tl(I) (thallium), Pb(II) (lead), and Bi(III) (bismuth) complexes a 6s electron pair exists. The inert pair effect is the tendency of this pair of electrons to resist oxidation due to a relativistic contraction of the 6s orbital.
Other effects
Additional phenomena commonly caused by relativistic effects are the following:
- The effect of relativistic effects on metallophilic interactions is uncertain. Although Runeberg et al. (1999) calculated an attractive effect, Wan et al. (2021) instead calculated a repulsive effect.
- The stability of gold and platinum anions in compounds such as caesium auride.
- The slightly reduced reactivity of francium compared with caesium.
- About 10% of the lanthanide contraction is attributed to the relativistic mass of high-velocity electrons and the smaller Bohr radius that results.
See also
- Ionization energy
- Electronegativity
- Electron affinity
- Quantum mechanics
- Relativistic quantum mechanics
References
- Pekka Pyykkö (January 2012). "Relativistic Effects in Chemistry: More Common Than You Thought". Annual Review of Physical Chemistry. 63 (1): 45–64. Bibcode:2012ARPC...63...45P. doi:10.1146/annurev-physchem-032511-143755. PMID 22404585.
- Kleppner, Daniel (1999). "A short history of atomic physics in the twentieth century" (PDF). Reviews of Modern Physics. 71 (2): S78–S84. Bibcode:1999RvMPS..71...78K. doi:10.1103/RevModPhys.71.S78. Archived from the original (PDF) on 2016-03-03. Retrieved 2012-07-17.
- Kaldor, U.; Wilson, Stephen (2003). Theoretical Chemistry and Physics of Heavy and Superheavy Elements. Dordrecht, Netherlands: Kluwer Academic Publishers. p. 4. ISBN 978-1-4020-1371-3.
- Kaldor, U.; Wilson, Stephen (2003). Theoretical Chemistry and Physics of Heavy and Superheavy Elements. Dordrecht, Netherlands: Kluwer Academic Publishers. p. 2. ISBN 978-1-4020-1371-3.
- Gupta, V. P. (22 October 2015). Principles and Applications of Quantum Chemistry. Elsevier Science. ISBN 978-0-12-803478-1. Retrieved 2024-01-07.
- Swirles, B. (1935). "The Relativistic Self-Consistent Field". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 152 (877): 625–649. Bibcode:1935RSPSA.152..625S. doi:10.1098/rspa.1935.0211.
- Dirac, P. A. M. (1929). "Quantum Mechanics of Many-Electron Systems". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 123 (792): 714–733. Bibcode:1929RSPSA.123..714D. doi:10.1098/rspa.1929.0094. JSTOR 95222.
- ^ Pyykkö, Pekka (1988). "Relativistic effects in structural chemistry". Chemical Reviews. 88 (3): 563–594. doi:10.1021/cr00085a006.
- Erwin Schrödinger (1926). "Über das Verhältnis der Heisenberg-Born-Jordanschen Quantenmechanik zu der meinem" (PDF). Annalen der Physik (in German). 384 (8). Leipzig: 734–756. Bibcode:1926AnP...384..734S. doi:10.1002/andp.19263840804. Archived from the original (PDF) on 2008-12-17.
- Kaldor, U.; Wilson, Stephen, eds. (2003). Theoretical Chemistry and Physics of Heavy and Superheavy Elements. Dordrecht, Netherlands: Kluwer Academic Publishers. p. 17. ISBN 978-1-4020-1371-3.
- ^ Norrby, Lars J. (1991). "Why is mercury liquid? Or, why do relativistic effects not get into chemistry textbooks?". Journal of Chemical Education. 68 (2): 110. Bibcode:1991JChEd..68..110N. doi:10.1021/ed068p110.
- Pitzer, Kenneth S. (1979). "Relativistic effects on chemical properties" (PDF). Accounts of Chemical Research. 12 (8): 271–276. doi:10.1021/ar50140a001. S2CID 95601322.
- Calvo, Florent; Pahl, Elke; Wormit, Michael; Schwerdtfeger, Peter (2013). "Evidence for Low-Temperature Melting of Mercury owing to Relativity". Angewandte Chemie International Edition. 52 (29): 7583–7585. doi:10.1002/anie.201302742. ISSN 1521-3773. PMID 23780699.
- Howes2013-06-21T00:00:00+01:00, Laura. "Relativity behind mercury's liquidity". Chemistry World. Retrieved 2022-01-21.
{{cite web}}: CS1 maint: numeric names: authors list (link) - Zehnacker, A (Jun 1, 1987). "Experimental study of the cold mercury dimer". Journal of Chemical Physics. 86 (11): 6565–6566. Bibcode:1987JChPh..86.6565Z. doi:10.1063/1.452401.
- Pyykkö, Pekka; Desclaux, Jean Paul (1979). "Relativity and the periodic system of elements". Accounts of Chemical Research. 12 (8): 276. doi:10.1021/ar50140a002.
- Addison, C. C. (1984). The chemistry of the liquid alkali metals. Wiley. p. 7. ISBN 9780471905080.
- Ahuja, Rajeev; Blomqvist, Anders; Larsson, Peter; Pyykkö, Pekka; Zaleski-Ejgierd, Patryk (2011). "Relativity and the Lead-Acid Battery". Physical Review Letters. 106 (1): 018301. arXiv:1008.4872. Bibcode:2011PhRvL.106a8301A. doi:10.1103/PhysRevLett.106.018301. PMID 21231773. S2CID 39265906.
- Runeberg, Nino; Schütz, Martin; Werner, Hans-Joachim (1999). "The aurophilic attraction as interpreted by local correlation methods". J. Chem. Phys. 110 (15): 7210–7215. Bibcode:1999JChPh.110.7210R. doi:10.1063/1.478665.
- Wan, Qingyun; Yang, Jun; To, Wai-Pong; Che, Chi-Ming (2021-01-05). "Strong metal–metal Pauli repulsion leads to repulsive metallophilicity in closed-shell d 8 and d 10 organometallic complexes". Proceedings of the National Academy of Sciences. 118 (1): e2019265118. Bibcode:2021PNAS..11819265W. doi:10.1073/pnas.2019265118. ISSN 0027-8424. PMC 7817198. PMID 33372160.
- Jansen, Martin (December 2005). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences. 7 (12): 1464–1474. Bibcode:2005SSSci...7.1464J. doi:10.1016/j.solidstatesciences.2005.06.015.
- "IYPT 2019 Elements 087: Francium: Not the most reactive group 1 element". Explorations of everyday chemical compounds. November 6, 2019.
- Andreev, S. V.; Letokhov, V. S.; Mishin, V. I. (21 September 1987). "Laser resonance photoionization spectroscopy of Rydberg levels in Fr". Physical Review Letters. 59 (12): 1274–1276. Bibcode:1987PhRvL..59.1274A. doi:10.1103/PhysRevLett.59.1274. PMID 10035190.
- Pyykko, Pekka (1 May 1988). "Relativistic effects in structural chemistry". Chemical Reviews. 88 (3): 563–594. doi:10.1021/cr00085a006.
Further reading
- P. A. Christiansen; W. C. Ermler; K. S. Pitzer. Relativistic Effects in Chemical Systems. Annual Review of Physical Chemistry 1985, 36, 407–432. doi:10.1146/annurev.pc.36.100185.002203
 (ordinate) is equal to
(ordinate) is equal to  but as
but as  , the
, the 
 are the
are the  ), which is given by
), which is given by

 is the
is the  for a 1s electron, where v is its
for a 1s electron, where v is its 

 where
where  is the
is the  . Substituting into the equation above and solving for
. Substituting into the equation above and solving for  gives
gives



 results in
results in  . This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, and as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
. This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, and as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.