 | |
| Clinical data | |
|---|---|
| Other names | Roglethimide; Pyridoglutethimide |
| Routes of administration | By mouth |
| Drug class | Aromatase inhibitor |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H14N2O2 |
| Molar mass | 218.256 g·mol |
| 3D model (JSmol) | |
SMILES
| |
Rogletimide, also known as pyridoglutethimide, is a medication which was never marketed. It is related in chemical structure to the sedative/hypnotic drug glutethimide, but instead has pharmacological activity as a selective aromatase inhibitor similar to the related drug aminoglutethimide and has no significant sedative-hypnotic effect. This makes it potentially useful in the treatment of breast cancer, and with fewer side effects than aminoglutethimide, but its lower potency caused it to be unsuccessful in clinical trials.
| Generation | Medication | Dosage | % inhibition | Class | IC50 |
|---|---|---|---|---|---|
| First | Testolactone | 250 mg 4x/day p.o. | ? | Type I | ? |
| 100 mg 3x/week i.m. | ? | ||||
| Rogletimide | 200 mg 2x/day p.o. 400 mg 2x/day p.o. 800 mg 2x/day p.o. |
50.6% 63.5% 73.8% |
Type II | ? | |
| Aminoglutethimide | 250 mg mg 4x/day p.o. | 90.6% | Type II | 4,500 nM | |
| Second | Formestane | 125 mg 1x/day p.o. 125 mg 2x/day p.o. 250 mg 1x/day p.o. |
72.3% 70.0% 57.3% |
Type I | 30 nM |
| 250 mg 1x/2 weeks i.m. 500 mg 1x/2 weeks i.m. 500 mg 1x/1 week i.m. |
84.8% 91.9% 92.5% | ||||
| Fadrozole | 1 mg 1x/day p.o. 2 mg 2x/day p.o. |
82.4% 92.6% |
Type II | ? | |
| Third | Exemestane | 25 mg 1x/day p.o. | 97.9% | Type I | 15 nM |
| Anastrozole | 1 mg 1x/day p.o. 10 mg 1x/day p.o. |
96.7–97.3% 98.1% |
Type II | 10 nM | |
| Letrozole | 0.5 mg 1x/day p.o. 2.5 mg 1x/day p.o. |
98.4% 98.9%–>99.1% |
Type II | 2.5 nM | |
| Footnotes: = In postmenopausal women. = Type I: Steroidal, irreversible (substrate-binding site). Type II: Nonsteroidal, reversible (binding to and interference with the cytochrome P450 heme moiety). = In breast cancer homogenates. Sources: See template. | |||||
Synthesis
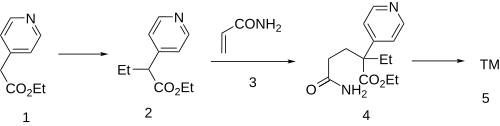
Base catalyzed alkylation of ethyl 4-pyridylacetate (1) with iodoethane gives ethyl 2-(4-pyridyl)butyrate (2). Base catalyzed conjugate addition of the carbanion to acrylamide (3) gives (4). The last step is an intramolecular cyclization to rogletimide (5).
References
- ^ Frederick A. Luzzio (17 May 2019). Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry. Elsevier Science. pp. 361–. ISBN 978-0-12-815676-6.
- Vanden Bossche HV, Moereels H, Koymans LM (1994). "Aromatase inhibitors--mechanisms for non-steroidal inhibitors". Breast Cancer Research and Treatment. 30 (1): 43–55. doi:10.1007/bf00682740. PMID 7949204. S2CID 24414161.
- MacNeill FA, Jones AL, Jacobs S, Lønning PE, Powles TJ, Dowsett M (October 1992). "The influence of aminoglutethimide and its analogue rogletimide on peripheral aromatisation in breast cancer". British Journal of Cancer. 66 (4): 692–7. doi:10.1038/bjc.1992.339. PMC 1977412. PMID 1419608.
- Boss, Aileen M.; W. Clissold, Derek; Mann, John; Markson, Andrew J.; Thickitt, Christopher P. (1989). "A concise synthesis of racemic pyridoglutethimide and its resolution using chiral stationary phase HPLC". Tetrahedron. 45 (18): 6011–6016. doi:10.1016/S0040-4020(01)89128-6.
- Derek W. Clissold, John Mann, Christopher P. Thickitt, US5112976 (1992 to National Research Development Corporation).
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |