| |
| Names | |
|---|---|
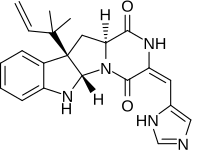
| Preferred IUPAC name (3E,5aS,10bR,11aS)-3--10b-(2-methylbut-3-en-2-yl)-6,10b,11,11a-tetrahydro-2H-pyrazinopyrroloindole-1,4(3H,5aH)-dione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H23N5O2 |
| Molar mass | 389.5 g/mol |
| Appearance | White to off-white solid |
| Solubility in water | Soluble in ethanol, methanol, DMF or DMSO |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.
Roquefortine C is a cyclodipeptide mycotoxin derived from the diketopiperazine cyclo(Trp-dehydro-His) and is a relatively common fungal metabolite produced by a number of Penicillium species. It is also considered one of the most important fungal contaminants of carbonated beverages, beer, wine, meats, cheese and bread. At high doses roquefortine C is classified as a toxic compound. Although it is a potent neurotoxin at high doses, at low concentrations of 0.05 to 1.47 mg/kg that occur in domestic cheeses, it was found to be "safe for the consumer". The mechanisms underlying its toxicity and metabolism have been investigated by studying its interaction with mammalian cytochrome P450 enzymes. In addition to these toxic properties, roquefortine C reportedly possesses bacteriostatic activity against gram-positive bacteria, but only in those organisms containing haemoproteins.
Roquefortine C contains the unusual E-dehydrohistidine moiety, a system that typically undergoes facile isomerization under acidic, basic, or photochemical conditions to isoroquefortine C, the 3,12 double-bond Z-isomer of roquefortine C.

However isoroquefortine C is not a natural product and in contrast to roquefortine C does not bind iron. Both have been synthesised.
Related compounds
References
- Borthwick AD (2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- Kokkonen M, Jestoi M, Rizzo A (2005). "The effect of substrate on mycotoxin production of selected Penicillium strains". International Journal of Food Microbiology. 99 (2): 207–14. doi:10.1016/j.ijfoodmicro.2004.08.014. PMID 15734568.
- Borthwick AD, Da Costa NC (2017). "2,5-Diketopiperazines in Food and Beverages: Taste and Bioactivity". Critical Reviews in Food Science and Nutrition. 57 (4): 718–742. doi:10.1080/10408398.2014.911142. PMID 25629623. S2CID 1334464.
- ^ Aninat C, Hayashi Y, André F, Delaforge M (July 2001). "Molecular requirements for inhibition of cytochrome P450 activities by roquefortine". Chemical Research in Toxicology. 14 (9): 1259–1265. doi:10.1021/tx015512l. PMID 11559041.
- SCBT. "Roquefortine - A potent neurotoxin produced most notably by Penicillium species".
{{cite journal}}: Cite journal requires|journal=(help) - EPA. "Penicillium roqueforti Final Risk Assessment".
{{cite journal}}: Cite journal requires|journal=(help) - Finoli C, Vecchio A, Galli A, Dragoni I (February 2001). "Roquefortine C occurrence in blue cheese". J. Food Prot. 64 (2): 246–51. doi:10.4315/0362-028x-64.2.246. PMID 11271775.
- Kopp-Holtwiesche B, Rehm HJ (December 1989). "Antimicrobial action of roquefortine". Journal of Environmental Pathology, Toxicology and Oncology. 10 (1–2): 41–44. PMID 2231314.
- Aninat C, Andre F, Delaforge M (April 2005). "Oxidative metabolism by P450 and function coupling to efflux systems: modulation of mycotoxin toxicity". Food Additives and Contaminants. 22 (4): 361–368. doi:10.1080/02652030500073287. PMID 16019806. S2CID 9880652.
- ^ Shangguan N, Hehre WJ, Ohlinger WS, Beavers MP, Joullie MM (April 2008). "The total synthesis of roquefortine C and a rationale for the thermodynamic stability of isoroquefortine C over roquefortine C". Journal of the American Chemical Society. 130 (19): 6281–6287. doi:10.1021/ja800067q. PMID 18412344.