| |||
| |||
| Names | |||
|---|---|---|---|
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) |
| ||
| ChemSpider | |||
| PubChem CID | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | Se2S6 | ||
| Molar mass | 350.30 g·mol | ||
| Appearance | orange needles | ||
| Density | 2.44 g/cm | ||
| Melting point | 121.5 °C (250.7 °F; 394.6 K) | ||
| Solubility | soluble in carbon disulfide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
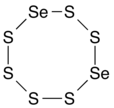
Selenium hexasulfide is a chemical compound with the chemical formula Se2S6. Its molecular structure is an 8-membered ring, consisting of two selenium and six sulfur atoms (diselenacyclooctasulfane), analogous to the S8 ring, an allotrope of sulfur (cyclooctasulfur or cyclooctasulfane), and other 8-membered rings of selenium sulfides with formula SenS8−n.
There are several isomers depending on the relative placement of the selenium atoms in the ring: 1,2-diselenacyclooctasulfane (with the two Se atoms adjacent), 1,3-diselenacyclooctasulfane, 1,4-diselenacyclooctasulfane, and 1,5-diselenacyclooctasulfane (with the Se atoms opposite). It is an oxidizing agent.
The 1,2 isomer can be prepared by reaction of chlorosulfanes and dichlorodiselane with potassium iodide in carbon disulfide. The reaction produces also cyclooctaselenium Se8 and all other eight-member cyclic selenium sulfides, except selenacyclooctasulfane SeS7, and several six- and seven-membered rings.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–81, ISBN 0-8493-0594-2
- ^ Risto S. Laitinen, Pentti Pekone, Yrjö Hiltunen and Tapanin A. Pakkanen (1989), "The S NMR spestroscopic Identification of Heterocyclic Selenium Sulfides Prepared by the Reactions of Chlorosulfanes and Dichlorodiselane with Potassium Iodide". Acta Chemica Scandinavica, volume 43, pages 436-440. doi:10.3891/acta.chem.scand.43-0436
- Arto Maaninen, Tristram Chivers, Masood Parvez, Jarkko Pietikäinen, and Risto S. Laitinen (1999), "Syntheses of THF Solutions of SeX2 (X = Cl, Br) and a New Route to Selenium Sulfides SenS8−n (n = 1−5): X-ray Crystal Structures of SeCl2(tht)2 and SeCl2·tmtu". Inorganic Chemistry, volume 38, issue 18, pages 4093–4097. doi:10.1021/ic981430h
| Selenium compounds | |
|---|---|
| Se(−II) | |
| Se(0,I) | |
| Se(I) | |
| Se(II) | |
| Se(IV) | |
| Se(VI) | |
| Se(IV,VI) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |