Campaniform sensilla are a class of mechanoreceptors found in insects, which respond to local stress and strain within the animal's cuticle. Campaniform sensilla function as proprioceptors that detect mechanical load as resistance to muscle contraction, similar to mammalian Golgi tendon organs. Sensory feedback from campaniform sensilla is integrated in the control of posture and locomotion.
Structure
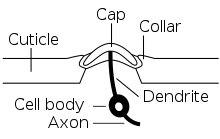
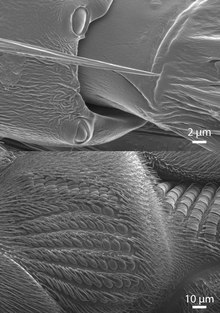
Each campaniform sensillum consists of a flexible dome, which is embedded in a spongy socket within the cuticle and innervated by the dendrites of a single bipolar sensory neuron (see schematic cross-section). Campaniform sensilla are often oval-shaped with long axes of about 5-10 μm (see SEM).
Campaniform sensilla are distributed across the body surface of many insects. The fruit fly Drosophila melanogaster, for example, has over 680 sensilla. Campaniform sensilla are located in regions where stress is likely to be high, including on the legs, antennae, wings, and halteres. Sensilla may occur alone, but sensilla with similar orientations are often grouped together.
Campaniform sensilla on legs
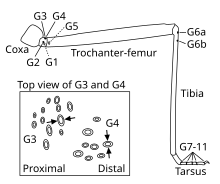
On the legs, groups of campaniform sensilla are located close to the joints on all segments except for the coxa (see leg schematic), with most sensilla located on the proximal trochanter. The number and location of sensilla on the legs varies little across individuals of the same species, and homologous groups of sensilla can be found across species.
Campaniform sensilla on wings and halteres

Campaniform sensilla typically occur on both sides of the wing (see wing schematic). The exact number and placement varies widely across species, likely mirroring differences in flight behavior. However, across species, most campaniform sensilla are found near the wing base. Computational models predict that this is an optimal location for sensing body rotations during flight, with sensing performance being robust to external perturbations and sensor loss.
In Diptera such as Drosophila, the highest density of campaniform sensilla is found at the base of the modified hind-wings, the halteres (see haltere schematic).
Function
Response properties
When cuticular deformations compress a campaniform sensillum, the socket edges (collar) indent the cuticular cap. This squeezes the dendritic tip of the sensory neuron and opens its mechanotransduction channels (from the TRP family), which leads to the generation of action potentials that are transmitted to the ventral nerve cord, the insect analogue to the vertebrate spinal cord.
The activity of campaniform sensilla was first recorded by John William Sutton Pringle in the late 1930s. Pringle also determined that the oval shape of many sensilla makes them directionally selective – they respond best to compression along their short axis. Thus, even neighboring sensilla may have very different sensitivities to strain depending on their orientation in the cuticle. For example, stick insects possess two groups of campaniform sensilla on the dorsal side of their legs' trochanter whose short axes are oriented perpendicularly to one another (see inset in leg schematic). As a result, one group (G3) responds when the leg is bent upwards, whereas the other group (G4) responds when the leg is bent downwards. Round campaniform sensilla can be sensitive in all directions or show directional sensitivity if the cap is asymmetrically coupled with the surrounding collar.
The activity of campaniform sensilla may be slowly-adapting (tonic), signaling the magnitude of cuticular deformation, and/or rapidly adapting (phasic), signaling the rate of cuticular deformation. Based on their responses to white noise stimuli, campaniform sensilla may also be described more generally as signaling two features that approximate the derivative of each other. This suggests that the neural response properties of the sensilla are rather generic, and that functional specialization arises primarily from how the sensilla are embedded in the cuticle. In addition, activity adapts to constant loads and shows hysteresis (history dependence) in response to cyclic loading.
Campaniform sensilla project directly to motor neurons and to various interneurons, which integrate their signals with signals from other proprioceptors. In this way, campaniform sensilla activity can affect the magnitude and timing of muscle contractions.
Function of leg campaniform sensilla
Campaniform sensilla on the legs are activated during standing and walking. Their sensory feedback is thought to reinforce muscle activity during the stance phase and to contribute to inter-leg coordination, much like sensory feedback from mammalian Golgi tendon organs. Feedback from leg campaniform sensilla is also important for the control of kicking and jumping.
Function of wing and haltere campaniform sensilla
Campaniform sensilla on the wings and halteres are activated as these structures oscillate back and forth during flight, with the phase of activation depending on the placement of the sensilla. The campaniform sensilla on the wing encode the wing's aerodynamic and inertial forces, whereas sensilla on the base of the haltere are thought to encode Coriolis forces induced by body rotation during flight, allowing the structure to function as a gyroscope. Feedback from wing and haltere campaniform sensilla is thought to mediate compensatory reflexes to maintain equilibrium during flight.
Computational models
To better understand the function of campaniform sensilla, computational models that mimic their response properties are being developed for use in simulations and robotics. On robotic legs, the models can filter input from engineered strain sensors "campaniform-sensilla-style" in real time. One advantage of this bio-inspired filtering is that it enables adaptation to load over time (see above), which makes strain sensors essentially self-calibrating to different loads carried by the robot.
References
- ^ Zill SN, Schmitz J, Chaudhry S, Büschges A (September 2012). "Force encoding in stick insect legs delineates a reference frame for motor control". Journal of Neurophysiology. 108 (5): 1453–72. doi:10.1152/jn.00274.2012. PMC 3774582. PMID 22673329.
- Zill SN, Chaudhry S, Büschges A, Schmitz J (November 2013). "Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps". Arthropod Structure & Development. 42 (6): 455–467. doi:10.1016/j.asd.2013.10.001. PMID 24126203.
- Duysens J, Clarac F, Cruse H (January 2000). "Load-regulating mechanisms in gait and posture: comparative aspects". Physiological Reviews. 80 (1): 83–133. doi:10.1152/physrev.2000.80.1.83. PMID 10617766.
- Tuthill JC, Azim E (March 2018). "Proprioception". Current Biology. 28 (5): R194–R203. doi:10.1016/j.cub.2018.01.064. PMID 29510103.
- ^ Zill S, Schmitz J, Büschges A (July 2004). "Load sensing and control of posture and locomotion". Arthropod Structure & Development. 33 (3): 273–86. doi:10.1016/j.asd.2004.05.005. PMID 18089039.
- Tuthill JC, Wilson RI (October 2016). "Mechanosensation and Adaptive Motor Control in Insects". Current Biology. 26 (20): R1022–R1038. doi:10.1016/j.cub.2016.06.070. PMC 5120761. PMID 27780045.
- ^ Dinges GF, Chockley AS, Bockemühl T, Ito K, Blanke A, Büschges A (July 2020). "Location and arrangement of campaniform sensilla in Drosophila melanogaster". The Journal of Comparative Neurology. 529 (4): 905–925. doi:10.1002/cne.24987. PMID 32678470.
- ^ Agrawal, Sweta; Grimaldi, David; Fox, Jessica L. (2017-03-01). "Haltere morphology and campaniform sensilla arrangement across Diptera". Arthropod Structure & Development. 46 (2): 215–229. doi:10.1016/j.asd.2017.01.005. ISSN 1467-8039. PMID 28161605.
- ^ Aiello, Brett R.; Stanchak, Kathryn E.; Weber, Alison I.; Deora, Tanvi; Sponberg, Simon; Brunton, Bingni W. (2021-06-24). "Spatial distribution of campaniform sensilla mechanosensors on wings: Form, function, and phylogeny". Current Opinion in Insect Science. 48: 8–17. doi:10.1016/j.cois.2021.06.002. ISSN 2214-5745. PMID 34175464.
- ^ Harris CM, Dinges GF, Haberkorn A, Gebehart C, Büschges A, Zill SN (September 2020). "Gradients in mechanotransduction of force and body weight in insects". Arthropod Structure & Development. 58: 100970. doi:10.1016/j.asd.2020.100970. PMID 32702647.
- Weber, Alison I.; Daniel, Thomas L.; Brunton, Bingni W. (2021-08-11). Webb, Barbara (ed.). "Wing structure and neural encoding jointly determine sensing strategies in insect flight". PLOS Computational Biology. 17 (8): e1009195. Bibcode:2021PLSCB..17E9195W. doi:10.1371/journal.pcbi.1009195. ISSN 1553-7358. PMC 8382179. PMID 34379622.
- Spinola SM, Chapman KM (1975-09-01). "Proprioceptive indentation of the campaniform sensilla of cockroach legs". Journal of Comparative Physiology. 96 (3): 257–272. doi:10.1007/BF00612698. ISSN 1432-1351. S2CID 8017950.
- Liang X, Madrid J, Saleh HS, Howard J (January 2011). "NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells". Cytoskeleton. 68 (1): 1–7. doi:10.1002/cm.20493. PMC 3048163. PMID 21069788.
- Pringle JW (1938). "Proprioception in insects I. A new type of mechanical receptor from the palps of the cockroach". Journal of Experimental Biology. 15 (1): 101–113. doi:10.1242/jeb.15.1.101.
- Pringle JW (1938). "Proprioception in insects II. The action of the campaniform sensilla on the legs". Journal of Experimental Biology. 15 (1): 114–131. doi:10.1242/jeb.15.1.114.
- Dickinson MH (1992-08-01). "Directional Sensitivity and Mechanical Coupling Dynamics of Campaniform Sensilla During Chordwise Deformations of the Fly Wing". Journal of Experimental Biology. 169 (1): 221–233. doi:10.1242/jeb.169.1.221. ISSN 0022-0949.
- Zill SN, Büschges A, Schmitz J (August 2011). "Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus". Journal of Comparative Physiology A: Neuroethology, Sensory, Neural & Behavioral Physiology. 197 (8): 851–67. doi:10.1007/s00359-011-0647-4. PMID 21544617. S2CID 20865515.
- ^ Ridgel AL, Frazier SF, DiCaprio RA, Zill SN (April 2000). "Encoding of forces by cockroach tibial campaniform sensilla: implications in dynamic control of posture and locomotion". Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology. 186 (4): 359–74. doi:10.1007/s003590050436. PMID 10798724. S2CID 12306005.
- ^ Dickerson, Bradley H.; Fox, Jessica L.; Sponberg, Simon (2020-11-28). "Functional diversity from generic encoding in insect campaniform sensilla". Current Opinion in Physiology. 19: 194–203. doi:10.1016/j.cophys.2020.11.004. ISSN 2468-8673.
- Dinges, Gesa F.; Bockemühl, Till; Iacoviello, Francesco; Shearing, Paul R.; Büschges, Ansgar; Blanke, Alexander (May 2022). "Ultra high-resolution biomechanics suggest that substructures within insect mechanosensors decisively affect their sensitivity". Journal of the Royal Society Interface. 19 (190): 20220102. doi:10.1098/rsif.2022.0102. ISSN 1742-5662. PMC 9065962. PMID 35506211.
- Phelps, Jasper S.; Hildebrand, David Grant Colburn; Graham, Brett J.; Kuan, Aaron T.; Thomas, Logan A.; Nguyen, Tri M.; Buhmann, Julia; Azevedo, Anthony W.; Sustar, Anne; Agrawal, Sweta; Liu, Mingguan; Shanny, Brendan L.; Funke, Jan; Tuthill, John C.; Lee, Wei-Chung Allen (2021-02-04). "Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy". Cell. 184 (3): 759–774.e18. doi:10.1016/j.cell.2020.12.013. ISSN 0092-8674. PMC 8312698. PMID 33400916.
- Gebehart, Corinna; Schmidt, Joachim; Büschges, Ansgar (2021-05-01). "Distributed processing of load and movement feedback in the premotor network controlling an insect leg joint". Journal of Neurophysiology. 125 (5): 1800–1813. doi:10.1152/jn.00090.2021. ISSN 0022-3077. PMID 33788591. S2CID 232480916.
- Keller, Bridget R.; Duke, Elizabeth R.; Amer, Ayman S.; Zill, Sasha N. (August 2007). "Tuning posture to body load: decreases in load produce discrete sensory signals in the legs of freely standing cockroaches". Journal of Comparative Physiology A: Neuroethology, Sensory, Neural & Behavioral Physiology. 193 (8): 881–891. doi:10.1007/s00359-007-0241-y. ISSN 0340-7594. PMID 17541783. S2CID 44044892.
- ^ Pearson KG (1972). "Central Programming and Reflex Control of Walking in the Cockroach". Journal of Experimental Biology. 56 (1): 173–193. doi:10.1242/jeb.56.1.173. ISSN 0022-0949.
- Zill SN, Chaudhry S, Büschges A, Schmitz J (November 2015). "Force feedback reinforces muscle synergies in insect legs". Arthropod Structure & Development. 44 (6 Pt A): 541–53. doi:10.1016/j.asd.2015.07.001. PMID 26193626.
- Zill SN, Keller BR, Duke ER (May 2009). "Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking". Journal of Neurophysiology. 101 (5): 2297–304. doi:10.1152/jn.00056.2009. PMID 19261716. S2CID 14691776.
- Dallmann CJ, Hoinville T, Dürr V, Schmitz J (December 2017). "A load-based mechanism for inter-leg coordination in insects". Proceedings. Biological Sciences. 284 (1868): 20171755. doi:10.1098/rspb.2017.1755. PMC 5740276. PMID 29187626.
- Pearson KG (January 2008). "Role of sensory feedback in the control of stance duration in walking cats". Brain Research Reviews. Networks in Motion. 57 (1): 222–7. doi:10.1016/j.brainresrev.2007.06.014. PMID 17761295. S2CID 23068577.
- Ekeberg O, Pearson K (December 2005). "Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition". Journal of Neurophysiology. 94 (6): 4256–68. doi:10.1152/jn.00065.2005. PMID 16049149. S2CID 7185159.
- Burrows M, Pflüger HJ (1988-07-01). "Positive feedback loops from proprioceptors involved in leg movements of the locust". Journal of Comparative Physiology A. 163 (4): 425–440. doi:10.1007/BF00604897. S2CID 25848693.
- Norman AP (August 1996). "Proprioceptive feedback in locust kicking and jumping during maturation". Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology. 179 (2): 195–205. doi:10.1007/BF00222786. PMID 8765558. S2CID 2312224.
- DICKINSON, MICHAEL H. (1990-07-01). "Comparison of Encoding Properties of Campaniform Sensilla on the Fly Wing". Journal of Experimental Biology. 151 (1): 245–261. doi:10.1242/jeb.151.1.245. ISSN 0022-0949.
- Pringle, John William Sutton; Gray, James (1948-11-02). "The gyroscopic mechanism of the halteres of Diptera". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 233 (602): 347–384. Bibcode:1948RSPTB.233..347P. doi:10.1098/rstb.1948.0007.
- Dickinson MH (May 1999). van Leeuwen JL (ed.). "Haltere-mediated equilibrium reflexes of the fruit fly, Drosophila melanogaster". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 354 (1385): 903–16. doi:10.1098/rstb.1999.0442. PMC 1692594. PMID 10382224.
- Fayyazuddin A, Dickinson MH (October 1999). "Convergent mechanosensory input structures the firing phase of a steering motor neuron in the blowfly, Calliphora". Journal of Neurophysiology. 82 (4): 1916–26. doi:10.1152/jn.1999.82.4.1916. PMID 10515981. S2CID 15194097.
- Szczecinski, Nicholas S.; Zill, Sasha N.; Dallmann, Chris J.; Quinn, Roger D. (2020). "Modeling the Dynamic Sensory Discharges of Insect Campaniform Sensilla". In Vouloutsi, Vasiliki; Mura, Anna; Tauber, Falk; Speck, Thomas; Prescott, Tony J.; Verschure, Paul F. M. J. (eds.). Biomimetic and Biohybrid Systems. Lecture Notes in Computer Science. Vol. 12413. Cham: Springer International Publishing. pp. 342–353. doi:10.1007/978-3-030-64313-3_33. ISBN 978-3-030-64313-3. S2CID 230716960.
- Goldsmith, C. A.; Szczecinski, N. S.; Quinn, R. D. (2020-09-14). "Neurodynamic modeling of the fruit fly Drosophila melanogaster". Bioinspiration & Biomimetics. 15 (6): 065003. doi:10.1088/1748-3190/ab9e52. ISSN 1748-3190. PMID 32924978. S2CID 219911361.
- ^ Zyhowski, William P.; Zill, Sasha N.; Szczecinski, Nicholas S. (2023). "Adaptive load feedback robustly signals force dynamics in robotic model of Carausius morosus stepping". Frontiers in Neurorobotics. 17. doi:10.3389/fnbot.2023.1125171. ISSN 1662-5218. PMC 9908954. PMID 36776993.