| |
| Names | |
|---|---|
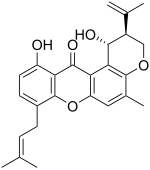
| Preferred IUPAC name (1R,2S)-1,11-Dihydroxy-5-methyl-8-(3-methylbut-2-en-1-yl)-2-(prop-1-en-2-yl)-2,3-dihydropyranoxanthene-12(1H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C25H26O5 |
| Molar mass | 406.478 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Shamixanthone is a chemical compound, classified as a prenylated xanthone, that has been isolated from several species of Aspergillus, including Aspergillus turkensis.
References
- Simpson, Thomas J. (2012). "Genetic and Biosynthetic Studies of the Fungal Prenylated Xanthone Shamixanthone and Related Metabolites in Aspergillus spp. Revisited". ChemBioChem. 13 (11): 1680–1688. doi:10.1002/cbic.201200014. PMID 22730213. S2CID 25478280.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |