| This article's lead section may be too short to adequately summarize the key points. Please consider expanding the lead to provide an accessible overview of all important aspects of the article. (October 2023) |
Photoperiod is the change of day length around the seasons. The rotation of the earth around its axis produces 24 hour changes in light (day) and dark (night) cycles on earth. The length of the light and dark in each phase varies across the seasons due to the tilt of the earth around its axis. The photoperiod defines the length of the light, for example a summer day the length of light could be 16 hours while the dark is 8 hours, whereas a winter day the length of day could be 8 hours, whereas the dark is 16 hours. Importantly, the seasons are different in the northern hemisphere than the southern hemisphere.
Photoperiodism is the physiological reaction of organisms to the length of light or a dark period. It occurs in plants and animals. Plant photoperiodism can also be defined as the developmental responses of plants to the relative lengths of light and dark periods. They are classified under three groups according to the photoperiods: short-day plants, long-day plants, and day-neutral plants.
In animals photoperiodism (sometimes called seasonality) is the suite of physiological changes that occur in response to changes in day length. This allows animals to respond to a temporally changing environment associated with changing seasons as the earth orbits the sun.
Plants

In 1920, W. W. Garner and H. A. Allard published their discoveries on photoperiodism and felt it was the length of daylight that was critical, but it was later discovered that the length of the night was the controlling factor. Photoperiodic flowering plants are classified as long-day plants or short-day plants even though night is the critical factor because of the initial misunderstanding about daylight being the controlling factor. Along with long-day plants and short-day plants, there are plants that fall into a "dual-day length category". These plants are either long-short-day plants (LSDP) or short-long-day plants (SLDP). LSDPs flower after a series of long days followed by short days whereas SLDPs flower after a series of short days followed by long days. Each plant has a different length critical photoperiod, or critical night length.
Many flowering plants (angiosperms) use a circadian rhythm together with photoreceptor protein, such as phytochrome or cryptochrome, to sense seasonal changes in night length, or photoperiod, which they take as signals to flower. In a further subdivision, obligate photoperiodic plants absolutely require a long or short enough night before flowering, whereas facultative photoperiodic plants are more likely to flower under one condition.
Phytochrome comes in two forms: Pr and Pfr. Red light (which is present during the day) converts phytochrome to its active form (Pfr) which then stimulates various processes such as germination, flowering or branching. In comparison, plants receive more far-red in the shade, and this converts phytochrome from Pfr to its inactive form, Pr, inhibiting germination. This system of Pfr to Pr conversion allows the plant to sense when it is night and when it is day. Pfr can also be converted back to Pr by a process known as dark reversion, where long periods of darkness trigger the conversion of Pfr. This is important in regards to plant flowering. Experiments by Halliday et al. showed that manipulations of the red-to far-red ratio in Arabidopsis can alter flowering. They discovered that plants tend to flower later when exposed to more red light, proving that red light is inhibitory to flowering. Other experiments have proven this by exposing plants to extra red-light in the middle of the night. A short-day plant will not flower if light is turned on for a few minutes in the middle of the night and a long-day plant can flower if exposed to more red-light in the middle of the night.
Cryptochromes are another type of photoreceptor that is important in photoperiodism. Cryptochromes absorb blue light and UV-A. Cryptochromes entrain the circadian clock to light. It has been found that both cryptochrome and phytochrome abundance relies on light and the amount of cryptochrome can change depending on day-length. This shows how important both of the photoreceptors are in regards to determining day-length.
Modern biologists believe that it is the coincidence of the active forms of phytochrome or cryptochrome, created by light during the daytime, with the rhythms of the circadian clock that allows plants to measure the length of the night. Other than flowering, photoperiodism in plants includes the growth of stems or roots during certain seasons and the loss of leaves. Artificial lighting can be used to induce extra-long days.
Long-day plants

Long-day plants flower when the night length falls below their critical photoperiod. These plants typically flower during late spring or early summer as days are getting longer. In the northern hemisphere, the longest day of the year (summer solstice) is on or about 21 June. After that date, days grow shorter (i.e. nights grow longer) until 21 December (the winter solstice). This situation is reversed in the southern hemisphere (i.e., longest day is 21 December and shortest day is 21 June).
Some long-day obligate plants are:
Some long-day facultative plants are:
Short-day plants
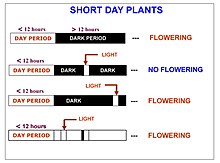
Short-day (also called long-night) plants flower when the night lengths exceed their critical photoperiod. They cannot flower under short nights or if a pulse of artificial light is shone on the plant for several minutes during the night; they require a continuous period of darkness before floral development can begin. Natural nighttime light, such as moonlight or lightning, is not of sufficient brightness or duration to interrupt flowering.
Short-day plants flower as days grow shorter (and nights grow longer) after September 21st in the northern hemisphere, which is during summer or fall. The length of the dark period required to induce flowering differs among species and varieties of a species.
Photoperiodism affects flowering by inducing the shoot to produce floral buds instead of leaves and lateral buds.
Some short-day facultative plants are:
- Kenaf ( Hibiscus cannabinus)
- Marijuana (Cannabis)
- Cotton (Gossypium)
- Rice (Oryza)
- Sorghum (Sorghum bicolor)
- Green gram (Mung bean, Vigna radiata)
- Soybeans (Glycine max)
Day-neutral plants
Day-neutral plants, such as cucumbers, roses, tomatoes, and Ruderalis (autoflowering cannabis) do not initiate flowering based on photoperiodism. Instead, they may initiate flowering after attaining a certain overall developmental stage or age, or in response to alternative environmental stimuli, such as vernalisation (a period of low temperature).
Animals
Daylength, and thus knowledge of the season of the year, is vital to many animals. A number of biological and behavioural changes are dependent on this knowledge. Together with temperature changes, photoperiod provokes changes in the color of fur and feathers, migration, entry into hibernation, sexual behaviour, and even the resizing of organs.
In insects, sensitivity to photoperiod has been proven to be initiated by photoreceptors located in the brain. Photoperiod can affect insects at different life stages, serving as an environmental cue for physiological processes such as diapause induction and termination, and seasonal morphs. In the water strider Aquarius paludum, for instance, photoperiod conditions during nymphal development have been shown to trigger seasonal changes in wing frequency and also induce diapause, although the threshold critical day lengths for the determination of both traits diverged by about an hour. In Gerris buenoi, another water strider species, photoperiod has also been shown to be the cause of wing polyphenism, although the specific daylengths changed between species, suggesting that phenotypic plasticity in response to photoperiod has evolved even between relatively closely related species.
The singing frequency of birds such as the canary depends on the photoperiod. In the spring, when the photoperiod increases (more daylight), the male canary's testes grow. As the testes grow, more androgens are secreted and song frequency increases. During autumn, when the photoperiod decreases (less daylight), the male canary's testes regress and androgen levels drop dramatically, resulting in decreased singing frequency. Not only is singing frequency dependent on the photoperiod but the song repertoire is also. The long photoperiod of spring results in a greater song repertoire. Autumn's shorter photoperiod results in a reduction in song repertoire. These behavioral photoperiod changes in male canaries are caused by changes in the song center of the brain. As the photoperiod increases, the high vocal center (HVC) and the robust nucleus of the archistriatum (RA) increase in size. When the photoperiod decreases, these areas of the brain regress.
Mammals
In mammals, daylength is registered in the suprachiasmatic nucleus (SCN), which is informed by retinal light-sensitive ganglion cells, which are not involved in vision. The information travels through the retinohypothalamic tract (RHT). In most species the hormone melatonin is produced by the pineal gland only during the hours of darkness, influenced by the light input through the RHT and by innate circadian rhythms. This hormonal signal, combined with outputs from the SCN inform the rest of the body about the time of day, and the length of time that melatonin is secreted is how the time of year is perceived.
Many mammals, particularly those inhabiting temperate and polar regions, exhibit a remarkable degree of seasonality in response to changes in daylight hours(photoperiod). This seasonality manifests in a broad spectrum of behaviors and physiology, including hibernation, seasonal migrations, and coat color changes. A prime example of the adaptation to photoperiods is the seasonal coat color (SCC) species. These animals undergo molting, transforming from dark summer fur to white coat in winter, that provides crucial camouflage in snowy environments.
Humans
The view has been expressed that humans' seasonality is largely believed to be evolutionary baggage.. Human birth rate varies throughout the year, and the peak month of births appears to vary by latitude. Seasonality in human birth rate appears to have largely decreased since the industrial revolution.
Other organisms
Photoperiodism has also been demonstrated in other organisms besides plants and animals. The fungus Neurospora crassa as well as the dinoflagellate Lingulodinium polyedra and the unicellular green alga Chlamydomonas reinhardtii have been shown to display photoperiodic responses.
See also
- Chronobiology
- Circadian clock
- Circadian rhythm
- Florigen
- Photobiology
- Seasonal Breeder
- Scotobiology
- Epigenetics of plant growth and development § Photoperiodism
References
- ^ Mauseth JD (2003). Botany : An Introduction to Plant Biology (3rd ed.). Sudbury, MA: Jones and Bartlett Learning. pp. 422–27. ISBN 978-0-7637-2134-3.
- ^ Capon B (2005). Botany for Gardeners (2nd ed.). Portland, OR: Timber Publishing. pp. 148–51. ISBN 978-0-88192-655-2.
- Hamner KC, Bonner J (1938). "Photoperiodism in relation to hormones as factors in floral initiation and development" (PDF). Botanical Gazette. 100 (2): 388–431. doi:10.1086/334793. JSTOR 2471641. S2CID 84084837.
- Hamner KC (1940). "Interrelation of light and darkness in photoperiodic induction". Botanical Gazette. 101 (3): 658–87. doi:10.1086/334903. JSTOR 2472399. S2CID 83682483.
- Taiz L, Zeiger E, Møller I, Murphy A (2015). Plant Physiology and Development (Sixth ed.). Sunderland, MA: Sinauer Associates, Inc. ISBN 978-1-60535-353-1.
- Fankhauser C (April 2001). "The phytochromes, a family of red/far-red absorbing photoreceptors". The Journal of Biological Chemistry. 276 (15): 11453–11456. doi:10.1074/jbc.R100006200. PMID 11279228.
- Casal JJ, Candia AN, Sellaro R (June 2014). "Light perception and signalling by phytochrome A". Journal of Experimental Botany. 65 (11): 2835–2845. doi:10.1093/jxb/ert379. hdl:11336/4338. PMID 24220656.
- Lin C (May 2000). "Photoreceptors and regulation of flowering time". Plant Physiology. 123 (1): 39–50. doi:10.1104/pp.123.1.39. PMC 1539253. PMID 10806223.
- Chamovitz D (2013). What A Plant Knows. Scientific American. pp. 17–18. ISBN 978-0-374-28873-0.
- Lin C, Todo T (2005). "The cryptochromes". Genome Biology. 6 (5): 220. doi:10.1186/gb-2005-6-5-220. PMC 1175950. PMID 15892880.
- Mockler T, Yang H, Yu X, Parikh D, Cheng YC, Dolan S, Lin C (February 2003). "Regulation of photoperiodic flowering by Arabidopsis photoreceptors". Proceedings of the National Academy of Sciences of the United States of America. 100 (4): 2140–2145. Bibcode:2003PNAS..100.2140M. doi:10.1073/pnas.0437826100. PMC 149972. PMID 12578985.
- Andrés F, Galbraith DW, Talón M, Domingo C (October 2009). "Analysis of PHOTOPERIOD SENSITIVITY5 sheds light on the role of phytochromes in photoperiodic flowering in rice". Plant Physiology. 151 (2): 681–690. doi:10.1104/pp.109.139097. PMC 2754645. PMID 19675157.
- Starr C, Taggart R, Evers C, Starr L (2013). Plant Structure and Function. Vol. 4 (13th ed.). Brooks/Cole. p. 517. ISBN 978-1-111-58068-1.
- Gooley T (2010-03-30). The Natural Navigator. Random House. ISBN 978-0-7535-2311-7.
- BSCS Biology (9 ed.). BSCS. 2002. p. 519. ISBN 978-0-7872-9008-5.
- Jones HG (1992). Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. Cambridge University Press. p. 225. ISBN 978-0-521-42524-7.
- Purcell LC, Salmeron M, Ashlock L (2014). "Chapter 2" (PDF). Arkansas Soybean Production Handbook - MP197. Little Rock, Arkansas: University of Arkansas Cooperative Extension Service. pp. 5–7. Retrieved 21 February 2016.
- Meneely P (2014). Genetic Analysis: Genes, Genomes, and Networks in Eukaryotes (2nd ed.). Oxford University Press. p. 373. ISBN 978-0-19-968126-6.
- Claret J (1966). "Recherche du centre photorecepteur lors de l'induction de la diapause chez Pieris brassicae L.". Comptes Rendus de l'Académie des Sciences. 262: 553–556.
- Bowen MF, Saunders DS, Bollenbacher WE, Gilbert LI (September 1984). "In vitro reprogramming of the photoperiodic clock in an insect brain-retrocerebral complex". Proceedings of the National Academy of Sciences of the United States of America. 81 (18): 5881–4. Bibcode:1984PNAS...81.5881B. doi:10.1073/pnas.81.18.5881. PMC 391816. PMID 6592591.
- Saunders DS (2012). "Insect photoperiodism: seeing the light". Physiological Entomology. 37 (3): 207–218. doi:10.1111/j.1365-3032.2012.00837.x. S2CID 85249708.
- Harada T, Numata H (1993). "Two Critical Day Lengths for the Determination of Wing Forms and the Induction of Adult Diapause in the Water Strider, Aquarius paludum". Naturwissenschaften. 80 (9): 430–432. Bibcode:1993NW.....80..430H. doi:10.1007/BF01168342. S2CID 39616943.
- Gudmunds E, Narayanan S, Lachivier E, Duchemin M, Khila A, Husby A (April 2022). "Photoperiod controls wing polyphenism in a water strider independently of insulin receptor signalling". Proceedings. Biological Sciences. 289 (1973): 20212764. doi:10.1098/rspb.2021.2764. PMC 9043737. PMID 35473377.
- Nelson RJ (2005). An Introduction to Behavioral Endocrinology. Sunderland, MA: Sinauer Associates. p. 189.
- Zimova, Marketa; Hackländer, Klaus; Good, Jeffrey M.; Melo-Ferreira, José; Alves, Paulo Célio; Mills, L. Scott (August 2018). "Function and underlying mechanisms of seasonal colour moulting in mammals and birds: what keeps them changing in a warming world?". Biological Reviews. 93 (3): 1478–1498. doi:10.1111/brv.12405. hdl:10216/118423. ISSN 1464-7931. PMID 29504224.
- Foster R, Williams R (5 December 2009). "Extra-retinal photo receptors" (Interview). Science Show. ABC Radio National. Retrieved 2010-05-28.
...we have the evolutionary baggage of showing seasonality but we're not entirely sure what the mechanism is.
- ^ Martinez-Bakker, Micaela; Bakker, Kevin M.; King, Aaron A.; Rohani, Pejman (2014-05-22). "Human birth seasonality: latitudinal gradient and interplay with childhood disease dynamics". Proceedings of the Royal Society B: Biological Sciences. 281 (1783): 20132438. doi:10.1098/rspb.2013.2438. ISSN 0962-8452. PMC 3996592. PMID 24695423.
- Wehr, Thomas A. (August 2001). "Photoperiodism in Humans and Other Primates: Evidence and Implications". Journal of Biological Rhythms. 16 (4): 348–364. doi:10.1177/074873001129002060. ISSN 0748-7304. PMID 11506380. S2CID 25886221.
- Tan Y, Merrow M, Roenneberg T. Photoperiodism in Neurospora crassa. J Biol Rhythms. 2004 Apr;19(2):135-43. https://doi.org/10.1177/0748730404263015. PMID 15038853.
- Suzuki, L., Johnson, C. Photoperiodic control of germination in the unicell Chlamydomonas. Naturwissenschaften 89, 214–220 (2002). https://doi.org/10.1007/s00114-002-0302-6
- Ivonne Balzer, Rüdiger Hardeland ,Photoperiodism and Effects of Indoleamines in a Unicellular Alga, Gonyaulax polyedra.Science253,795-797(1991).https://doi.org/10.1126/science.1876838
Further reading
- Fosket DE (1994). Plant Growth & Development, A Molecular Approach. San Diego: Academic Press. p. 495.
- Thomas B, Vince-Prue D (1997). Photoperiodism in plants (2nd ed.). Academic Press.
| Biological rhythms | ||
|---|---|---|
| Internal rhythms |  | |
| External cycles | ||
| Fields | ||
| See also | ||