| |
| Names | |
|---|---|
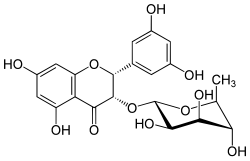
| IUPAC name (2R,3S)-2-(3,5-dihydroxyphenyl)-5,7-dihydroxy-3-oxy-2,3-dihydrochromen-4-one | |
| Other names Isoastilbin B | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H22O11 |
| Molar mass | 450.39 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Smitilbin is a flavanonol, a type of flavonoid. It is a rhamnoside that can be isolated in Smilax glabra (Chinaroot, sarsaparilla).
Uses
Smitilbin could be used for preventing immunological hepatocyte damage.
Related compounds

Neosmitilbin is a stereoisomer of smitilbin.
References
- Smitilbin on metabolomics.jp
- A Flavonol Glycoside from Smilax glabra, Ting Chen, Jian Xin Li, Yu Cai, Qiang Xu, Chinese Chemical Letters, Vol. 13, No 6, 2002, pages 537-538
- A new flavanone isolated from rhizoma smilacis glabrae and the structural requirements of its derivatives for preventing immunological hepatocyte damage. Planta Med 65(1):56-9 (1999), T Chen, J Li, J Cao, Q Xu, K Komatsu and T Namba
| Flavanonols and their glycosides | |
|---|---|
| 3-Hydroxyflavanones: |
|
| O-methylated flavanonols | |
| dihydroflavonol 3-O-glycosides | |
| Glycosides |
|
| Acetylated glycosides | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |