| This article may be too technical for most readers to understand. Relevant discussion may be found on the talk page. Please help improve it to make it understandable to non-experts, without removing the technical details. (February 2024) (Learn how and when to remove this message) |
In physics and chemistry, the spin quantum number is a quantum number (designated s) that describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. It has the same value for all particles of the same type, such as s = 1/2 for all electrons. It is an integer for all bosons, such as photons, and a half-odd-integer for all fermions, such as electrons and protons.
The component of the spin along a specified axis is given by the spin magnetic quantum number, conventionally written ms. The value of ms is the component of spin angular momentum, in units of the reduced Planck constant ħ, parallel to a given direction (conventionally labelled the z–axis). It can take values ranging from +s to −s in integer increments. For an electron, ms can be either ++1/2 or −+1/2 .
Nomenclature
The phrase spin quantum number refers to quantized spin angular momentum. The symbol s is used for the spin quantum number, and ms is described as the spin magnetic quantum number or as the z-component of spin sz.
Both the total spin and the z-component of spin are quantized, leading to two quantum numbers spin and spin magnet quantum numbers. The (total) spin quantum number has only one value for every elementary particle. Some introductory chemistry textbooks describe ms as the spin quantum number, and s is not mentioned since its value 1/2 is a fixed property of the electron; some even use the variable s in place of ms.
The two spin quantum numbers and are the spin angular momentum analogs of the two orbital angular momentum quantum numbers and .
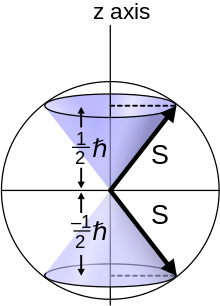
Spin quantum numbers apply also to systems of coupled spins, such as atoms that may contain more than one electron. Capitalized symbols are used: S for the total electronic spin, and mS or MS for the z-axis component. A pair of electrons in a spin singlet state has S = 0, and a pair in the triplet state has S = 1, with mS = −1, 0, or +1. Nuclear-spin quantum numbers are conventionally written I for spin, and mI or MI for the z-axis component.
The name "spin" comes from a geometrical spinning of the electron about an axis, as proposed by Uhlenbeck and Goudsmit. However, this simplistic picture was quickly realized to be physically unrealistic, because it would require the electrons to rotate faster than the speed of light. It was therefore replaced by a more abstract quantum-mechanical description.
History
See also: Spin (physics) § HistoryDuring the period between 1916 and 1925, much progress was being made concerning the arrangement of electrons in the periodic table. In order to explain the Zeeman effect in the Bohr atom, Sommerfeld proposed that electrons would be based on three 'quantum numbers', n, k, and m, that described the size of the orbit, the shape of the orbit, and the direction in which the orbit was pointing. Irving Langmuir had explained in his 1919 paper regarding electrons in their shells, "Rydberg has pointed out that these numbers are obtained from the series . The factor two suggests a fundamental two-fold symmetry for all stable atoms." This configuration was adopted by Edmund Stoner, in October 1924 in his paper 'The Distribution of Electrons Among Atomic Levels' published in the Philosophical Magazine.
The qualitative success of the Sommerfeld quantum number scheme failed to explain the Zeeman effect in weak magnetic field strengths, the anomalous Zeeman effect. In December 1924, Wolfgang Pauli showed that the core electron angular momentum was not related to the effect as had previously been assumed. Rather he proposed that only the outer "light" electrons determined the angular momentum and he hypothesized that this required a fourth quantum number with a two-valuedness. This fourth quantum number became the spin magnetic quantum number.
Electron spin
Main article: Spin (physics)A spin- 1 /2 particle is characterized by an angular momentum quantum number for spin s = 1 /2. In solutions of the Schrödinger-Pauli equation, angular momentum is quantized according to this number, so that magnitude of the spin angular momentum is
The hydrogen spectrum fine structure is observed as a doublet corresponding to two possibilities for the z-component of the angular momentum, where for any given direction z:
whose solution has only two possible z-components for the electron. In the electron, the two different spin orientations are sometimes called "spin-up" or "spin-down".
The spin property of an electron would give rise to magnetic moment, which was a requisite for the fourth quantum number.
The magnetic moment vector of an electron spin is given by:
where is the electron charge, is the electron mass, and is the electron spin g-factor, which is approximately 2.0023. Its z-axis projection is given by the spin magnetic quantum number according to:
where is the Bohr magneton.
When atoms have even numbers of electrons the spin of each electron in each orbital has opposing orientation to that of its immediate neighbor(s). However, many atoms have an odd number of electrons or an arrangement of electrons in which there is an unequal number of "spin-up" and "spin-down" orientations. These atoms or electrons are said to have unpaired spins that are detected in electron spin resonance.
Nuclear spin
Atomic nuclei also have spins. The nuclear spin I is a fixed property of each nucleus and may be either an integer or a half-integer. The component mI of nuclear spin parallel to the z–axis can have (2I + 1) values I, I–1, ..., –I. For example, a N nucleus has I = 1, so that there are 3 possible orientations relative to the z–axis, corresponding to states mI = +1, 0 and −1.
The spins I of different nuclei are interpreted using the nuclear shell model. Even-even nuclei with even numbers of both protons and neutrons, such as C and O, have spin zero. Odd mass number nuclei have half-integer spins, such as 3/ 2 for Li, 1 /2 for C and 5/ 2 for O, usually corresponding to the angular momentum of the last nucleon added. Odd-odd nuclei with odd numbers of both protons and neutrons have integer spins, such as 3 for B, and 1 for N. Values of nuclear spin for a given isotope are found in the lists of isotopes for each element. (See isotopes of oxygen, isotopes of aluminium, etc. etc.)
Detection of spin
When lines of the hydrogen spectrum are examined at very high resolution, they are found to be closely spaced doublets. This splitting is called fine structure, and was one of the first experimental evidences for electron spin. The direct observation of the electron's intrinsic angular momentum was achieved in the Stern–Gerlach experiment.
Stern–Gerlach experiment
Main article: Stern-Gerlach experimentThe theory of spatial quantization of the spin moment of the momentum of electrons of atoms situated in the magnetic field needed to be proved experimentally. In 1922 (two years before the theoretical description of the spin was created) Otto Stern and Walter Gerlach observed it in the experiment they conducted.
Silver atoms were evaporated using an electric furnace in a vacuum. Using thin slits, the atoms were guided into a flat beam and the beam sent through an in-homogeneous magnetic field before colliding with a metallic plate. The laws of classical physics predict that the collection of condensed silver atoms on the plate should form a thin solid line in the same shape as the original beam. However, the in-homogeneous magnetic field caused the beam to split in two separate directions, creating two lines on the metallic plate.
The phenomenon can be explained with the spatial quantization of the spin moment of momentum. In atoms the electrons are paired such that one spins upward and one downward, neutralizing the effect of their spin on the action of the atom as a whole. But in the valence shell of silver atoms, there is a single electron whose spin remains unbalanced.
The unbalanced spin creates spin magnetic moment, making the electron act like a very small magnet. As the atoms pass through the in-homogeneous magnetic field, the force moment in the magnetic field influences the electron's dipole until its position matches the direction of the stronger field. The atom would then be pulled toward or away from the stronger magnetic field a specific amount, depending on the value of the valence electron's spin. When the spin of the electron is ++ 1 /2 the atom moves away from the stronger field, and when the spin is −+ 1 /2 the atom moves toward it. Thus the beam of silver atoms is split while traveling through the in-homogeneous magnetic field, according to the spin of each atom's valence electron.
In 1927 Phipps and Taylor conducted a similar experiment, using atoms of hydrogen with similar results. Later scientists conducted experiments using other atoms that have only one electron in their valence shell: (copper, gold, sodium, potassium). Every time there were two lines formed on the metallic plate.
The atomic nucleus also may have spin, but protons and neutrons are much heavier than electrons (about 1836 times), and the magnetic dipole moment is inversely proportional to the mass. So the nuclear magnetic dipole momentum is much smaller than that of the whole atom. This small magnetic dipole was later measured by Stern, Frisch and Easterman.
Electron paramagnetic resonance
For atoms or molecules with an unpaired electron, transitions in a magnetic field can also be observed in which only the spin quantum number changes, without change in the electron orbital or the other quantum numbers. This is the method of electron paramagnetic resonance (EPR) or electron spin resonance (ESR), used to study free radicals. Since only the magnetic interaction of the spin changes, the energy change is much smaller than for transitions between orbitals, and the spectra are observed in the microwave region.
Relation to spin vectors
For a solution of either the nonrelativistic Pauli equation or the relativistic Dirac equation, the quantized angular momentum (see angular momentum quantum number) can be written as: where
- is the quantized spin vector or spinor
- is the norm of the spin vector
- s is the spin quantum number associated with the spin angular momentum
- is the reduced Planck constant.
Given an arbitrary direction z (usually determined by an external magnetic field) the spin z-projection is given by
where ms is the magnetic spin quantum number, ranging from −s to +s in steps of one. This generates 2 s + 1 different values of ms.
The allowed values for s are non-negative integers or half-integers. Fermions have half-integer values, including the electron, proton and neutron which all have s = ++ 1 /2 . Bosons such as the photon and all mesons) have integer spin values.
Algebra
The algebraic theory of spin is a carbon copy of the angular momentum in quantum mechanics theory. First of all, spin satisfies the fundamental commutation relation: where is the (antisymmetric) Levi-Civita symbol. This means that it is impossible to know two coordinates of the spin at the same time because of the restriction of the uncertainty principle.
Next, the eigenvectors of and satisfy: where are the ladder (or "raising" and "lowering") operators.
Energy levels from the Dirac equation
In 1928, Paul Dirac developed a relativistic wave equation, now termed the Dirac equation, which predicted the spin magnetic moment correctly, and at the same time treated the electron as a point-like particle. Solving the Dirac equation for the energy levels of an electron in the hydrogen atom, all four quantum numbers including s occurred naturally and agreed well with experiment.
Total spin of an atom or molecule
For some atoms the spins of several unpaired electrons (s1, s2, ...) are coupled to form a total spin quantum number S. This occurs especially in light atoms (or in molecules formed only of light atoms) when spin–orbit coupling is weak compared to the coupling between spins or the coupling between orbital angular momenta, a situation known as L S coupling because L and S are constants of motion. Here L is the total orbital angular momentum quantum number.
For atoms with a well-defined S, the multiplicity of a state is defined as 2S + 1. This is equal to the number of different possible values of the total (orbital plus spin) angular momentum J for a given (L, S) combination, provided that S ≤ L (the typical case). For example, if S = 1, there are three states which form a triplet. The eigenvalues of Sz for these three states are +1ħ, 0, and −1ħ. The term symbol of an atomic state indicates its values of L, S, and J.
As examples, the ground states of both the oxygen atom and the dioxygen molecule have two unpaired electrons and are therefore triplet states. The atomic state is described by the term symbol P, and the molecular state by the term symbol Σ
g.
See also
References
- Pauling, Linus (1960). The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry. Ithaca, N.Y.: Cornell University Press. ISBN 0-8014-0333-2. OCLC 545520.
- "ISO 80000-10:2019". International Organization for Standardization. Retrieved 2019-09-15.
- Atkins, Peter; de Paula, Julio (2006). Atkins' Physical Chemistry (8th ed.). W.H. Freeman. p. 308. ISBN 0-7167-8759-8.
- Banwell, Colin N.; McCash, Elaine M. (1994). Fundamentals of Molecular Spectroscopy. McGraw-Hill. p. 135. ISBN 0-07-707976-0.
- ^ Perrino, Charles T.; Peterson, Donald L. (1989). "Another quantum number?". J. Chem. Educ. 66 (8): 623. Bibcode:1989JChEd..66..623P. doi:10.1021/ed066p623. ISSN 0021-9584.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th ed.). Prentice Hall. p. 333. ISBN 0-13-014329-4.
- Whitten, Kenneth W.; Galley, Kenneth D.; Davis, Raymond E. (1992). General Chemistry (4th ed.). Saunders College Publishing. p. 196. ISBN 0-03-072373-6.
- Karplus, Martin, and Porter, Richard Needham. Atoms and Molecules. United States, W.A. Benjamin, 1970.
- Halpern, Paul (2017-11-21). "Spin: The quantum property that should have been impossible". Forbes. Starts with a bang. Archived from the original on 2018-03-10. Retrieved 2018-03-10.
- Manjit Kumar, Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality, 2008.
- Langmuir, Irving (1919). "The arrangement of electrons in atoms and molecules". Journal of the Franklin Institute. 187 (3): 359–362. doi:10.1016/S0016-0032(19)91097-0.
- Giulini, Domenico (September 2008). "Electron spin or "classically non-describable two-valuedness"". Studies in History and Philosophy of Science Part B: Studies in History and Philosophy of Modern Physics. 39 (3): 557–578. arXiv:0710.3128. Bibcode:2008SHPMP..39..557G. doi:10.1016/j.shpsb.2008.03.005. hdl:11858/00-001M-0000-0013-13C8-1. S2CID 15867039.
- Wolfgang Pauli. Exclusion principle and quantum mechanics Nobel Lecture delivered on December 13th 1946 for the 1945 Nobel Prize in Physics.
- Atkins, Peter; de Paula, Julio (2006). Atkins' Physical Chemistry (8th ed.). W.H. Freeman. p. 515. ISBN 0-7167-8759-8.
- Cottingham, W.N.; Greenwood, D.A. (1986). An introduction to nuclear physics. Cambridge University Press. pp. 36, 57. ISBN 0-521-31960-9.
- David J. Griffiths, Introduction to Quantum Mechanics (book), Oregon, Reed College, 2018, 166 p. ISBN 9781107189638.
- ^ Merzbacher, E. (1998). Quantum Mechanics (3rd ed.). John Wiley. pp. 430–431. ISBN 0-471-88702-1.
- ^ Atkins, P.; de Paula, J. (2006). Physical Chemistry (8th ed.). W.H. Freeman. p. 352. ISBN 0-7167-8759-8.
External links
- Weiss, Michael (2001). "Full treatment of spin – including origins, evolution of spin theory, and details of the spin equations". Department of Mathematics. UC Riverside.
| Electron configuration | |
|---|---|
| Quantum numbers | |
| Ground-state configurations | |
| Electron filling | |
| Electron pairing | |
| Bonding participation | |
| Electron counting rules | |
 and
and  are the spin angular momentum analogs of the two
are the spin angular momentum analogs of the two  and
and  .
.
 . The factor two suggests a fundamental two-fold symmetry for all stable atoms." This
. The factor two suggests a fundamental two-fold symmetry for all stable atoms." This  configuration was adopted by
configuration was adopted by 


 is the
is the  is the
is the  is the
is the  according to:
according to:

 is the
is the  where
where
 is the quantized
is the quantized  is the
is the  is the
is the 

 where
where  is the (antisymmetric)
is the (antisymmetric)  and
and  satisfy:
satisfy:


 where
where  are the
are the