| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-Stannole | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
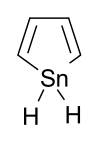
| Chemical formula | C4H6Sn | ||
| Molar mass | 172.802 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
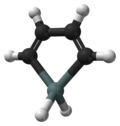
Stannole is an organotin compound with the formula (CH)4SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, with tin replacing the saturated carbon atom. Substituted derivatives, which have been synthesized, are also called stannoles.
1λ-Stannole has formula C4H4Sn, with no hydrogen on the tin atom, which is in the +2 oxidation state.
Examples
1,1-Dibutylstannole is a pale yellow oil prepared from 1,4-dilithio-1,3-butadiene and dibutyltin dichloride.
Reactions
1,1-Dimethyl-2,3,4,5-tetraphenyl-1H-stannole, for example, can be formed by the displacement reaction of 1,4-dilithio-1,2,3,4-tetraphenyl-1,3-butadiene and dimethyltin dichloride. 1,1-Disubstituted stannoles can be formed in the cycloaddition reaction of two acetylene molecules with an organotin molecule SnR2.
See also
References
- Dubac, Jacques; Laporterie, Andre; Manuel, Georges (1990). "Group 14 metalloles. 1. Synthesis, organic chemistry, and physicochemical data". Chemical Reviews. 90: 215–263. doi:10.1021/cr00099a008.
- "DTXSID70781612". pubchem.ncbi.nlm.nih.gov.
- Ashe, Arthur J.; Mahmoud, Samir. (1988). "1,4-Dilithio-1,3-butadienes". Organometallics. 7 (8): 1878. doi:10.1021/om00098a034.
- J.I.G. Cadogan; S.V. Ley; G. Pattenden; R.A. Raphael; C.W. Rees, eds. (1996). Dictionary of Organic Compounds. Vol. 3 (6 ed.). Chapman & Hall. p. 4219. ISBN 978-0-412-54090-5. Retrieved 2010-03-04.
- Davies, A.G. (2004). Organotin Chemistry (2 ed.). Wiley-VCH. p. 129. ISBN 978-3-527-31023-4. Retrieved 2010-03-04.