Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. The hallmarks of aging are the types of biochemical changes that occur in all organisms that experience biological aging and lead to a progressive loss of physiological integrity, impaired function and, eventually, death. They were first listed in a landmark paper in 2013 to conceptualize the essence of biological aging and its underlying mechanisms.
The following three premises for the interconnected hallmarks have been proposed:
- "their age-associated manifestation"
- "the acceleration of aging by experimentally accentuating them"
- "the opportunity to decelerate, stop, or reverse aging by therapeutic interventions on them"
Overview

Over time, almost all living organisms experience a gradual and irreversible increase in senescence and an associated loss of proper function of the bodily systems. As aging is the primary risk factor for major human diseases, including cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases, it is important to describe and classify the types of changes that it entails.
After a decade, the authors of the heavily cited original paper updated the set of proposed hallmarks in January 2023. In the new review, three new hallmarks have been added: macroautophagy, chronic inflammation and dysbiosis, totaling 12 proposed hallmarks.
The nine hallmarks of aging of the original paper are grouped into three categories as below:
Primary hallmarks (causes of damage)
- Genome instability
- Telomere shortening (or telomere attrition)
- Epigenetic alterations
- Loss of proteostasis
- macroautophagy
Antagonistic hallmarks (responses to damage)
Integrative hallmarks (culprits of the phenotype)
- Stem cell exhaustion
- Altered intercellular communication
- chronic inflammation
- dysbiosis
Primary hallmarks are the primary causes of cellular damage. Antagonistic hallmarks are antagonistic or compensatory responses to the manifestation of the primary hallmarks. Integrative hallmarks are the functional result of the previous two groups of hallmarks that lead to further operational deterioration associated with aging.
There are also proposed further hallmarks or underlying mechanisms that drive multiple of these hallmarks.
The hallmarks
Each hallmark was chosen to try to fulfill the following criteria:
- manifests during normal aging;
- experimentally increasing it accelerates aging;
- experimentally amending it slows the normal aging process and increases healthy lifespan.
These conditions are met to different extents by each of these hallmarks. The last criterion is not present in many of the hallmarks, as science has not yet found feasible ways to amend these problems in living organisms.
Genome instability
See also: Genome instabilityProper functioning of the genome is one of the most important prerequisites for the smooth functioning of a cell and the organism as a whole. Alterations in the genetic code have long been considered one of the main causal factors in aging. In multicellular organisms genome instability is central to carcinogenesis, and in humans it is also a factor in some neurodegenerative diseases such as amyotrophic lateral sclerosis or the neuromuscular disease myotonic dystrophy.
Abnormal chemical structures in the DNA are formed mainly through oxidative stress and environmental factors. A number of molecular processes work continuously to repair this damage. Unfortunately, the results are not perfect, and thus damage accumulates over time. Several review articles have shown that deficient DNA repair, allowing greater accumulation of DNA damages, causes premature aging; and that increased DNA repair facilitates greater longevity.
Telomere shortening
See also: Telomere § Association with aging
Telomeres are regions of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. They protect the terminal regions of chromosomal DNA from progressive degradation and ensure the integrity of linear chromosomes by preventing DNA repair systems from mistaking the ends of the DNA strand for a double strand break.
Telomere shortening is associated with aging, mortality and aging-related diseases. Normal aging is associated with telomere shortening in both humans and mice, and studies on genetically modified animal models suggest causal links between telomere erosion and aging. Leonard Hayflick demonstrated that a normal human fetal cell population will divide between 40 and 60 times in cell culture before entering a senescence phase. Each time a cell undergoes mitosis, the telomeres on the ends of each chromosome shorten slightly. Cell division will cease once telomeres shorten to a critical length. This is useful when uncontrolled cell proliferation (like in cancer) needs to be stopped, but detrimental when normally functioning cells are unable to divide when necessary.
An enzyme called telomerase elongates telomeres in gametes and stem cells. Telomerase deficiency in humans has been linked to several aging-related diseases related to loss of regenerative capacity of tissues. It has also been shown that premature aging in telomerase-deficient mice is reverted when telomerase is reactivated. The shelterin protein complex regulates telomerase activity in addition to protecting telomeres from DNA repair in eukaryotes.
Epigenomic alterations
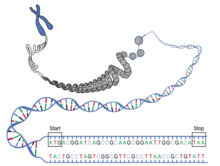
Out of all the genes that make up a genome, only a subset are expressed at any given time. The functioning of a genome depends both on the specific order of its nucleotides (genomic factors), and also on which sections of the DNA chain are spooled on histones and thus rendered inaccessible, and which ones are unspooled and available for transcription (epigenomic factors). Depending on the needs of the specific tissue type and environment that a given cell is in, histones can be modified to turn specific genes on or off as needed. The profile of where, when and to what extent these modifications occur (the epigenetic profile) changes with aging, turning useful genes off and unnecessary ones on, disrupting the normal functioning of the cell.
As an example, sirtuins are a type of protein deacetylases that promote the binding of DNA onto histones and thus turn unnecessary genes off. These enzymes use NAD as a cofactor. With aging, the level of NAD in cells decreases and so does the ability of sirtuins to turn off unneeded genes at the right time. Decreasing the activity of sirtuins has been associated with accelerated aging and increasing their activity has been shown to stave off several age-related diseases.
Loss of proteostasis
See also: Proteostasis § Proteostasis and agingProteostasis is the homeostatic process of maintaining all the proteins necessary for the functioning of the cell in their proper shape, structure and abundance. Protein misfolding, oxidation, abnormal cleavage or undesired post-translational modification can create dysfunctional or even toxic proteins or protein aggregates that hinder the normal functioning of the cell. Though these proteins are continually removed and recycled, formation of damaged or aggregated proteins increases with age, leading to a gradual loss of proteostasis. This can be slowed or suppressed by caloric restriction or by administration of rapamycin, both through inhibiting the mTOR pathway.
Deregulated nutrient sensing
See also: Nutrient sensingNutrient sensing is a cell's ability to recognize, and respond to, changes in the concentration of macronutrients such as glucose, fatty acids and amino acids. In times of abundance, anabolism is induced through various pathways, the most well-studied among them the mTOR pathway. When energy and nutrients are scarce, the AMPK receptor senses this and switches off mTOR to conserve resources.
In a growing organism, growth and cell proliferation are important and thus mTOR is upregulated. In a fully grown organism, mTOR-activating signals naturally decline during aging. It has been found that forcibly overactivating these pathways in grown mice leads to accelerated aging and increased incidence of cancer. mTOR inhibition methods like dietary restriction or administering rapamycin have been shown to be one of the most robust methods of increasing lifespan in worms, flies and mice.
Mitochondrial dysfunction
See also: Mitochondrion § Relationships to aging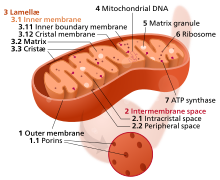
The mitochondrion is the powerhouse of the cell. Different human cells contain from several up to 2500 mitochondria, each one converting carbon (in the form of acetyl-CoA) and oxygen into energy (in the form of ATP) and carbon dioxide.
During aging, the efficiency of mitochondria tends to decrease. The reasons for this are still quite unclear, but several mechanisms are suspected: reduced biogenesis, accumulation of damage and mutations in mitochondrial DNA, oxidation of mitochondrial proteins, and defective quality control by mitophagy.
Dysfunctional mitochondria contribute to aging through interfering with intracellular signaling and triggering inflammatory reactions.
Cellular senescence
See also: Cellular senescenceUnder certain conditions, a cell will exit the cell cycle without dying, instead becoming dormant and ceasing its normal function. This is called cellular senescence. Senescence can be induced by several factors, including telomere shortening, DNA damage and stress. Since the immune system is programmed to seek out and eliminate senescent cells, it might be that senescence is one way for the body to rid itself of cells damaged beyond repair.
The links between cell senescence and aging are several:
- The proportion of senescent cells increases with age.
- Senescent cells secrete inflammatory markers which may contribute to aging.
- Clearance of senescent cells has been found to delay the onset of age-related disorders.
Stem cell exhaustion
See also: Adult stem cell § AgingStem cells are undifferentiated or partially differentiated cells with the unique ability to self-renew and differentiate into specialized cell types. For the first few days after fertilization, the embryo consists almost entirely of stem cells. As the fetus grows, the cells multiply, differentiate and assume their appropriate function within the organism. In adults, stem cells are mostly located in areas that undergo gradual wear (intestine, lung, mucosa, skin) or need continuous replenishment (red blood cells, immune cells, sperm cells, hair follicles).
Loss of regenerative ability is one of the most obvious consequences of aging. This is largely because the proportion of stem cells and the speed of their division gradually lowers over time. It has been found that stem cell rejuvenation can reverse some of the effects of aging at the organismal level.
Altered intercellular communication
See also: Cell signalingDifferent tissues and the cells they consist of need to orchestrate their work in a tightly controlled manner so that the organism as a whole can function. One of the main ways this is achieved is through excreting signal molecules into the blood where they make their way to other tissues, affecting their behavior. The profile of these molecules changes as we age.
One of the most prominent changes in cell signaling biomarkers is "inflammaging", the development of a chronic low-grade inflammation throughout the body with advanced age. The normal role of inflammation is to recruit the body's immune system and repair mechanisms to a specific damaged area for as long as the damage and threat are present. The constant presence of inflammation markers throughout the body wears out the immune system and damages healthy tissue.
It's also been found that senescent cells excrete a specific set of molecules called the SASP (Senescence-Associated Secretory Phenotype) which induce senescence in neighboring cells. Conversely, lifespan-extending manipulations targeting one tissue can slow the aging process in other tissues as well.
Further hallmarks
| This section needs expansion. You can help by adding to it. (March 2023) |
These may constitute further hallmarks or underlying mechanisms that drive multiple of these hallmarks.
- Resurrection of endogenous retroviruses could be "a hallmark and driving force of cellular senescence and tissue aging" as retroviruses in the human genomes can become awakened from dormant states and contribute to aging which can be blocked by neutralizing antibodies.
Alternative conceptual models

In 2014, other scientists have defined a slightly different conceptual model for aging, called 'The Seven Pillars of Aging', in which just three of the 'hallmarks of aging' are included (stem cells and regeneration, proteostasis, epigenetics). The seven pillars model highlights the interconnectedness between all of the seven pillars which is not highlighted in the nine hallmarks of aging model.
Links to other diseases or hallmarks
Authors of the original paper merged or linked various hallmarks of cancer with those of aging.
The authors also concluded that the hallmarks are not only interconnected among each other but also "to the recently proposed hallmarks of health, which include organizational features of spatial compartmentalization, maintenance of homeostasis, and adequate responses to stress".
See also
- Biomarkers of aging
- Senescence#Theories of aging
- Ageing#Biological basis
- Aging Brain
- Life extension#Strategies
- Negligible senescence
- Strategies for engineered negligible senescence
- Stem cell theory of aging
- Timeline of senescence research
References
- ^ López-Otín, Carlos; Blasco, Maria A.; Partridge, Linda; Serrano, Manuel; Kroemer, Guido (2013-06-06). "The Hallmarks of Aging". Cell. 153 (6): 1194–1217. doi:10.1016/j.cell.2013.05.039. ISSN 0092-8674. PMC 3836174. PMID 23746838.
- ^ López-Otín, Carlos; Blasco, Maria A.; Partridge, Linda; Serrano, Manuel; Kroemer, Guido (19 January 2023). "Hallmarks of aging: An expanding universe". Cell. 186 (2): 243–278. doi:10.1016/j.cell.2022.11.001. ISSN 0092-8674. PMID 36599349. S2CID 255394876. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- "New research extensively explores 12 distinctive aging traits". News-Medical.net. 5 January 2023. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- ^ Vijg, Jan; Suh, Yousin (2013-02-10). "Genome Instability and Aging". Annual Review of Physiology. 75 (1): 645–668. doi:10.1146/annurev-physiol-030212-183715. ISSN 0066-4278. PMID 23398157.
- Moskalev, Alexey; Shaposhnikov, Mikhail; Plyusnina, Ekaterina; Zhavoronkov, Alex; Budovsky, Arie; Yanai, Hagai; Fraifeld, Vadim (March 2013). "The role of DNA damage and repair in aging through the prism of Koch-like criteria". Ageing Research Reviews. 12 (2): 661–684. doi:10.1016/j.arr.2012.02.001. PMID 22353384. S2CID 26339878.
- Schmitt, Michael W.; Prindle, Marc J.; Loeb, Lawrence A. (2012-09-06). "Implications of genetic heterogeneity in cancer: Schmitt et al". Annals of the New York Academy of Sciences. 1267 (1): 110–116. doi:10.1111/j.1749-6632.2012.06590.x. PMC 3674777. PMID 22954224.
- De Bont, R. (2004-05-01). "Endogenous DNA damage in humans: a review of quantitative data". Mutagenesis. 19 (3): 169–185. doi:10.1093/mutage/geh025. ISSN 1464-3804. PMID 15123782.
- de Duve, Christian (2005-02-09). "The onset of selection". Nature. 433 (7026): 581–582. Bibcode:2005Natur.433..581D. doi:10.1038/433581a. ISSN 0028-0836. PMID 15703726. S2CID 4355530.
- Hoeijmakers, Jan H.J. (2009-10-08). "DNA Damage, Aging, and Cancer". New England Journal of Medicine. 361 (15): 1475–1485. doi:10.1056/NEJMra0804615. ISSN 0028-4793. PMID 19812404.
- Chakravarti, Deepavali; LaBella, Kyle A.; DePinho, Ronald A. (2021-01-14). "Telomeres: history, health, and hallmarks of aging". Cell. 184 (2): 306–322. doi:10.1016/j.cell.2020.12.028. PMC 8081271. PMID 33450206. S2CID 231607042.
- Hayflick, L.; Moorhead, P.S. (1961-05-15). "The serial cultivation of human diploid cell strains". Experimental Cell Research. 25 (3): 585–621. doi:10.1016/0014-4827(61)90192-6. PMID 13905658.
- Blasco, Maria A. (2005-08-01). "Telomeres and human disease: ageing, cancer and beyond". Nature Reviews Genetics. 6 (8): 611–622. doi:10.1038/nrg1656. ISSN 1471-0064. PMID 16136653. S2CID 14828121.
- Armanios, Mary; Alder, Jonathan K.; Parry, Erin M.; Karim, Baktiar; Strong, Margaret A.; Greider, Carol W. (2009-11-25). "Short Telomeres are Sufficient to Cause the Degenerative Defects Associated with Aging". The American Journal of Human Genetics. 85 (6): 823–832. doi:10.1016/j.ajhg.2009.10.028. PMC 2790562. PMID 19944403.
- Jaskelioff, Mariela; Muller, Florian L.; Paik, Ji-Hye; Thomas, Emily; Jiang, Shan; Adams, Andrew C.; Sahin, Ergun; Kost-Alimova, Maria; Protopopov, Alexei; Cadiñanos, Juan; Horner, James W. (2010-11-28). "Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice". Nature. 469 (7328): 102–106. doi:10.1038/nature09603. ISSN 1476-4687. PMC 3057569. PMID 21113150.
- Kouzarides, Tony (2007-02-23). "Chromatin Modifications and Their Function". Cell. 128 (4): 693–705. doi:10.1016/j.cell.2007.02.005. ISSN 0092-8674. PMID 17320507. S2CID 11691263.
- Siametis, Athanasios; Niotis, George; Garinis, George A. (2021-04-01). "DNA Damage and the Aging Epigenome". Journal of Investigative Dermatology. 141 (4): 961–967. doi:10.1016/j.jid.2020.10.006. ISSN 0022-202X. PMID 33494932. S2CID 231711205.
- Guarente, L. (2011-01-01). "Sirtuins, Aging, and Metabolism". Cold Spring Harbor Symposia on Quantitative Biology. 76: 81–90. doi:10.1101/sqb.2011.76.010629. ISSN 0091-7451. PMID 22114328.
- Haigis, Marcia C.; Sinclair, David A. (2010-01-01). "Mammalian Sirtuins: Biological Insights and Disease Relevance". Annual Review of Pathology: Mechanisms of Disease. 5 (1): 253–295. doi:10.1146/annurev.pathol.4.110807.092250. ISSN 1553-4006. PMC 2866163. PMID 20078221.
- Covarrubias, Anthony J.; Perrone, Rosalba; Grozio, Alessia; Verdin, Eric (2020-12-22). "NAD+ metabolism and its roles in cellular processes during ageing". Nature Reviews Molecular Cell Biology. 22 (2): 119–141. doi:10.1038/s41580-020-00313-x. PMC 7963035. PMID 33353981.
- Ottens, Franziska; Franz, André; Hoppe, Thorsten (2021-02-04). "Build-UPS and break-downs: metabolism impacts on proteostasis and aging". Cell Death & Differentiation. 28 (2): 505–521. doi:10.1038/s41418-020-00682-y. ISSN 1476-5403. PMC 7862225. PMID 33398091.
- Kirana, A.N.; Prafiantini, E.; Hardiany, N.S. (2021-02-22). "Protein intake and loss of proteostasis in the eldery [sic]". The Ukrainian Biochemical Journal. 93 (1): 30–39. doi:10.15407/ubj93.01.030.
- Klaips, Courtney L.; Jayaraj, Gopal Gunanathan; Hartl, F. Ulrich (2017-11-10). "Pathways of cellular proteostasis in aging and disease". Journal of Cell Biology. 217 (1): 51–63. doi:10.1083/jcb.201709072. ISSN 0021-9525. PMC 5748993. PMID 29127110.
- Yang, Ling; Licastro, Danilo; Cava, Edda; Veronese, Nicola; Spelta, Francesco; Rizza, Wanda; Bertozzi, Beatrice; Villareal, D.T.; Hotamisligil, G.S.; Holloszy, J.O.; Fontana, Luigi (2016-01-07). "Long-Term Calorie Restriction Enhances Cellular Quality-Control Processes in Human Skeletal Muscle". Cell Reports. 14 (3): 422–428. doi:10.1016/j.celrep.2015.12.042. hdl:10447/462955. PMID 26774472. S2CID 18786539.
- Blagosklonny, Mikhail V. (2013-12-15). "Aging is not programmed". Cell Cycle. 12 (24): 3736–3742. doi:10.4161/cc.27188. ISSN 1538-4101. PMC 3905065. PMID 24240128.
- Laplante, Mathieu; Sabatini, D.M. (2012-04-13). "mTOR Signaling in Growth Control and Disease". Cell. 149 (2): 274–293. doi:10.1016/j.cell.2012.03.017. PMC 3331679. PMID 22500797.
- Alers, S.; Loffler, A. S.; Wesselborg, S.; Stork, B. (2012-01-01). "Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks". Molecular and Cellular Biology. 32 (1): 2–11. doi:10.1128/MCB.06159-11. ISSN 0270-7306. PMC 3255710. PMID 22025673.
- Schumacher, Björn; van der Pluijm, Ingrid; Moorhouse, Michael J.; Kosteas, Theodore; Robinson, Andria Rasile; Suh, Yousin; Breit, Timo M.; van Steeg, Harry; Niedernhofer, Laura J.; van IJcken, Wilfred; Bartke, Andrzej (2008-08-15). Kim, Stuart K. (ed.). "Delayed and Accelerated Aging Share Common Longevity Assurance Mechanisms". PLOS Genetics. 4 (8): e1000161. doi:10.1371/journal.pgen.1000161. ISSN 1553-7404. PMC 2493043. PMID 18704162.
- Papadopoli, David; Boulay, Karine; Kazak, Lawrence; Pollak, Michael; Mallette, Frédérick; Topisirovic, Ivan; Hulea, Laura (2019-07-02). "mTOR as a central regulator of lifespan and aging". F1000Research. 8: 998. doi:10.12688/f1000research.17196.1. ISSN 2046-1402. PMC 6611156. PMID 31316753.
- Fontana, L.; Partridge, L.; Longo, V. D. (2010-04-16). "Extending Healthy Life Span--From Yeast to Humans". Science. 328 (5976): 321–326. Bibcode:2010Sci...328..321F. doi:10.1126/science.1172539. ISSN 0036-8075. PMC 3607354. PMID 20395504.
- Harrison, David E.; Strong, Randy; Sharp, Zelton Dave; Nelson, James F.; Astle, Clinton M.; Flurkey, Kevin; Nadon, Nancy L.; Wilkinson, J. Erby; Frenkel, Krystyna; Carter, Christy S.; Pahor, Marco (2009-07-16). "Rapamycin fed late in life extends lifespan in genetically heterogeneous mice". Nature. 460 (7253): 392–395. Bibcode:2009Natur.460..392H. doi:10.1038/nature08221. ISSN 0028-0836. PMC 2786175. PMID 19587680.
- "Строение клетки" [Scientific Digital Library: Cell structure]. Научная электронная библиотека (in Russian). Retrieved 23 March 2023.
- Sahin, Ergün; DePinho, Ronald A. (June 2012). "Axis of ageing: telomeres, p53 and mitochondria". Nature Reviews Molecular Cell Biology. 13 (6): 397–404. doi:10.1038/nrm3352. ISSN 1471-0072. PMC 3718675. PMID 22588366.
- Wang, Ke; Klionsky, Daniel J (March 2011). "Mitochondria removal by autophagy". Autophagy. 7 (3): 297–300. doi:10.4161/auto.7.3.14502. ISSN 1554-8627. PMC 3359476. PMID 21252623.
- Kroemer, Guido; Galluzzi, Lorenzo; Brenner, Catherine (January 2007). "Mitochondrial Membrane Permeabilization in Cell Death". Physiological Reviews. 87 (1): 99–163. doi:10.1152/physrev.00013.2006. ISSN 0031-9333. PMID 17237344.
- Raffaello, Anna; Rizzuto, Rosario (January 2011). "Mitochondrial longevity pathways". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1813 (1): 260–268. doi:10.1016/j.bbamcr.2010.10.007. PMID 20950653.
- Green, Douglas R.; Galluzzi, Lorenzo; Kroemer, Guido (2011-08-26). "Mitochondria and the Autophagy–Inflammation–Cell Death Axis in Organismal Aging". Science. 333 (6046): 1109–1112. Bibcode:2011Sci...333.1109G. doi:10.1126/science.1201940. ISSN 0036-8075. PMC 3405151. PMID 21868666.
- Bodnar, Andrea G.; Ouellette, Michel; Frolkis, Maria; Holt, Shawn E.; Chiu, Choy-Pik; Morin, Gregg B.; Harley, Calvin B.; Shay, Jerry W.; Lichtsteiner, Serge; Wright, Woodring E. (1998-01-16). "Extension of Life-Span by Introduction of Telomerase into Normal Human Cells". Science. 279 (5349): 349–352. Bibcode:1998Sci...279..349B. doi:10.1126/science.279.5349.349. ISSN 0036-8075. PMID 9454332.
- Collado, Manuel; Blasco, Maria A.; Serrano, Manuel (2007-07-27). "Cellular Senescence in Cancer and Aging". Cell. 130 (2): 223–233. doi:10.1016/j.cell.2007.07.003. ISSN 0092-8674. PMID 17662938. S2CID 18689141.
- Sagiv, Adi; Krizhanovsky, Valery (2013-12-01). "Immunosurveillance of senescent cells: the bright side of the senescence program". Biogerontology. 14 (6): 617–628. doi:10.1007/s10522-013-9473-0. ISSN 1573-6768. PMID 24114507. S2CID 2775067.
- Wang, Chunfang; Jurk, Diana; Maddick, Mandy; Nelson, Glyn; Martin‐Ruiz, Carmen; Zglinicki, Thomas Von (2009). "DNA damage response and cellular senescence in tissues of aging mice". Aging Cell. 8 (3): 311–323. doi:10.1111/j.1474-9726.2009.00481.x. ISSN 1474-9726. PMID 19627270. S2CID 9192359.
- Malaquin, Nicolas; Martinez, Aurélie; Rodier, Francis (2016-09-01). "Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype". Experimental Gerontology. 82: 39–49. doi:10.1016/j.exger.2016.05.010. ISSN 0531-5565. PMID 27235851. S2CID 207584394.
- Baker, Darren J.; Wijshake, Tobias; Tchkonia, Tamar; LeBrasseur, Nathan K.; Childs, Bennett G.; van de Sluis, Bart; Kirkland, James L.; van Deursen, Jan M. (2011-11-02). "Clearance of p16 Ink4a -positive senescent cells delays ageing-associated disorders". Nature. 479 (7372): 232–236. Bibcode:2011Natur.479..232B. doi:10.1038/nature10600. ISSN 1476-4687. PMC 3468323. PMID 22048312.
- Behrens, Axel; van Deursen, Jan M.; Rudolph, K. Lenhard; Schumacher, Björn (March 2014). "Impact of genomic damage and ageing on stem cell function". Nature Cell Biology. 16 (3): 201–207. doi:10.1038/ncb2928. ISSN 1476-4679. PMC 4214082. PMID 24576896.
- Rando, T.A.; Chang, H.Y. (2012-01-20). "Aging, Rejuvenation, and Epigenetic Reprogramming: Resetting the Aging Clock". Cell. 148 (1–2): 46–57. doi:10.1016/j.cell.2012.01.003. ISSN 0092-8674. PMC 3336960. PMID 22265401.
- Villeda, Saul A.; Luo, Jian; Mosher, Kira I.; Zou, Bende; Britschgi, Markus; et al. (31 August 2011). "The ageing systemic milieu negatively regulates neurogenesis and cognitive function". Nature. 477 (7362): 90–94. Bibcode:2011Natur.477...90V. doi:10.1038/nature10357. PMC 3170097. PMID 21886162.
- Loffredo, Francesco S.; Steinhauser, Matthew L.; Jay, Steven M.; Gannon, Joseph; Pancoast, James R.; et al. (9 May 2013). "Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy". Cell. 153 (4): 828–39. doi:10.1016/j.cell.2013.04.015. PMC 3677132. PMID 23663781.
- Panda, Alexander; Arjona, Alvaro; Sapey, Elizabeth; Bai, Fengwei; Fikrig, Erol; Montgomery, Ruth R.; Lord, Janet M.; Shaw, Albert C. (2009-06-22). "Human innate immunosenescence: causes and consequences for immunity in old age". Trends in Immunology. 30 (7): 325–333. doi:10.1016/j.it.2009.05.004. ISSN 1471-4906. PMC 4067971. PMID 19541535.
- Franceschi, Claudio; Bonafè, Massimiliano; Valensin, Silvana; Olivieri, Fabiola; Luca, Maria De; Ottaviani, Enzo; Benedictis, Giovanna De (2000). "Inflamm-aging: An Evolutionary Perspective on Immunosenescence". Annals of the New York Academy of Sciences. 908 (1): 244–254. Bibcode:2000NYASA.908..244F. doi:10.1111/j.1749-6632.2000.tb06651.x. ISSN 1749-6632. PMID 10911963. S2CID 1843716.
- Nelson, Glyn; Wordsworth, James; Wang, Chunfang; Jurk, Diana; Lawless, Conor; Martin‐Ruiz, Carmen; Zglinicki, Thomas von (2012). "A senescent cell bystander effect: senescence-induced senescence". Aging Cell. 11 (2): 345–349. doi:10.1111/j.1474-9726.2012.00795.x. ISSN 1474-9726. PMC 3488292. PMID 22321662.
- Lavasani, Mitra; Robinson, Andria R.; Lu, Aiping; Song, Minjung; Feduska, Joseph M.; Ahani, Bahar; Tilstra, Jeremy S.; Feldman, Chelsea H.; Robbins, Paul D.; Niedernhofer, Laura J.; Huard, Johnny (2012-01-03). "Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model". Nature Communications. 3 (1): 608. Bibcode:2012NatCo...3..608L. doi:10.1038/ncomms1611. ISSN 2041-1723. PMC 3272577. PMID 22215083.
- "Aging and Retroviruses". Science. 23 January 2023. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- Liu, Xiaoqian; Liu, Zunpeng; Wu, Zeming; Ren, Jie; Fan, Yanling; Sun, Liang; Cao, Gang; Niu, Yuyu; Zhang, Baohu; Ji, Qianzhao; Jiang, Xiaoyu; Wang, Cui; Wang, Qiaoran; Ji, Zhejun; Li, Lanzhu; Esteban, Concepcion Rodriguez; Yan, Kaowen; Li, Wei; Cai, Yusheng; Wang, Si; Zheng, Aihua; Zhang, Yong E.; Tan, Shengjun; Cai, Yingao; Song, Moshi; Lu, Falong; Tang, Fuchou; Ji, Weizhi; Zhou, Qi; Belmonte, Juan Carlos Izpisua; Zhang, Weiqi; Qu, Jing; Liu, Guang-Hui (19 January 2023). "Resurrection of endogenous retroviruses during aging reinforces senescence". Cell. 186 (2): 287–304.e26. doi:10.1016/j.cell.2022.12.017. ISSN 0092-8674. PMID 36610399. S2CID 232060038.
- Kennedy, Brian; Berger, Shelley (6 November 2014). "Geroscience: linking aging to chronic disease". Cell. 159 (4): 709–713. doi:10.1016/j.cell.2014.10.039. ISSN 1097-4172. PMC 4852871. PMID 25417146.
- Gems, David; de Magalhães, João Pedro (13 July 2021). "The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm". Ageing Research Reviews. 70: 101407. doi:10.1016/j.arr.2021.101407. ISSN 1568-1637. PMC 7611451. PMID 34271186.
- López-Otín, Carlos; Pietrocola, Federico; Roiz-Valle, David; Galluzzi, Lorenzo; Kroemer, Guido (3 January 2023). "Meta-hallmarks of aging and cancer". Cell Metabolism. 35 (1): 12–35. doi:10.1016/j.cmet.2022.11.001. ISSN 1550-4131. PMID 36599298. S2CID 255465457. Archived from the original on 17 February 2023. Retrieved 17 February 2023.
- López-Otín, Carlos; Kroemer, Guido (7 January 2021). "Hallmarks of Health". Cell. 184 (1): 33–63. doi:10.1016/j.cell.2020.11.034. ISSN 0092-8674. PMID 33340459. S2CID 229321394.