| |
| Names | |
|---|---|
| IUPAC name benzoate | |
| Other names Stovaine; Benzoic acid ester | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.375 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
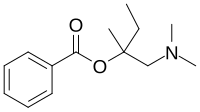
| Chemical formula | C14H21NO2 |
| Molar mass | 235.327 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Amylocaine was the first synthetic local anesthetic. It was synthesized and patented under the name Stovaine by Ernest Fourneau at the Pasteur Institute in 1903. It was used mostly in spinal anesthesia.
Synthesis
Amylocaine can be synthesized beginning with chloroacetone (1). Grignard reaction of chloroacetone with magnesium ethyl bromide gives 1-chloro-2-methyl-butan-2-ol (2). Heating with dimethylamine gives 1-(dimethylamino)-2-methylbutan-2-ol (3). These two steps can also be treated as interchangeable. Esterification with benzoyl chloride completed the synthesis of amylocaine (4).

See also
- Dimethylaminopivalophenone, an opioid with a similar chemical structure
Notes and references
- Fourneau, E. (1904). "Stovaïne, anesthésique local". Bulletin des sciences pharmacologiques. 10: 141–148.
- Debue-Barazer, Christine (2007). "Les Implications scientifiques et industrielles du succès de la Stovaïne : Ernest Fourneau (1872–1949) et la chimie des médicaments en France" Archived 2013-10-05 at the Wayback Machine. Gesnerus 64 (1-2): 24-53.
- ^ Quintard, Jean-Paul; Elissondo, Bernard; Jousseaume, Bernard (1984). "A Convenient Synthesis of N,N-Disubstituted Aminomethyltri-n-butylstannanes, Precursors of the Corresponding Lithium Reagents". Synthesis. 1984 (6): 495–498. doi:10.1055/s-1984-30879. ISSN 0039-7881. S2CID 95920500.
- ^ Fourneau, Ernest (1904). Comptes rendus hebdomadaires des séances de l'Académie des sciences. Vol. 138. Paris: Academy of Sciences, Centre national de la recherche scientifique (CNRS; French National Centre for Scientific Research). p. 767.
- Zernik, F (1905). "?". Chem. Zentralbl. 76 (1): 1029.
- DE169746C, "Patent number DE169746C" . Google Patents.
- DE169787C, "Patent number DE169787C" . Google Patents.
External links
- Smith, Maurice I.; Hatcher, Robert A. (January 1917). "A Contribution to the Pharmacology of Stovaine". Journal of Pharmacology and Experimental Therapeutics. 9 (4): 231–240.
- Ball, Christine M.; Westhorpe, Rod N. (2004). "Local Anaesthesia after Cocaine". Anaesthesia and Intensive Care. 32 (2): 157. doi:10.1177/0310057X0403200201. PMID 15957711.
| Local anesthetics (primarily sodium channel blockers) (N01B) | |||||||
|---|---|---|---|---|---|---|---|
| Esters by acid |
| ||||||
| Amides | |||||||
| Combinations | |||||||
| |||||||