| |
| Names | |
|---|---|
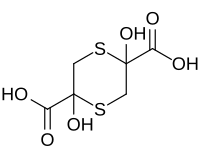
| IUPAC name 2,5-Dihydroxy-1,4-dithiane-2,5-dicarboxylic acid | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H8O6S2 |
| Molar mass | 240.24 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Sulfanegen is an experimental antidote for cyanide poisoning. It is being studied as a prodrug for 3-mercaptopyruvic acid (3-MP). 3-MP has been studied as a potential treatment for cyanide poisoning, but the half-life is too short for it to be clinically effective. Instead, alternative chemicals such as sulfanegen, the hemithioacetal cyclic dimer of 3-MP, are being evaluated that produce 3-MP in vivo to compensate for the short half-life of 3-MP itself.
Sulfanegen has been shown to be effective in animal studies. It is being studied as the disodium salt, sulfanegen sodium, and the triethanolamine salt, sulfanegen TEA. One advantage various sulfanegen formulations have over existing treatments for acute cyanide poisoning is that they might be administered by intramuscular injection or orally rather than by intravenous infusion.
References
- ^ "Scientists Discover Fast-Acting Cyanide Antidote". Medgadget. Dec 27, 2007. Retrieved 2015-07-12.
- Nagahara, N; Li, Q; Sawada, N (2003). "Do antidotes for acute cyanide poisoning act on mercaptopyruvate sulfurtransferase to facilitate detoxification?". Current Drug Targets. Immune, Endocrine and Metabolic Disorders. 3 (3): 198–204. doi:10.2174/1568008033340162. PMID 12871026.
- ^ Brenner, M; Kim, JG; Lee, J; Mahon, SB; Lemor, D; Ahdout, R; Boss, GR; Blackledge, W; Jann, L; Nagasawa, HT; Patterson, SE (2010). "Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity". Toxicology and Applied Pharmacology. 248 (3): 269–76. doi:10.1016/j.taap.2010.08.002. PMC 3382974. PMID 20705081.
- Chan, A; Crankshaw, DL; Monteil, A; Patterson, SE; Nagasawa, HT; Briggs, JE; Kozocas, JA; Mahon, SB; Brenner, M; Pilz, RB; Bigby, TD; Boss, GR (2011). "The combination of cobinamide and sulfanegen is highly effective in mouse models of cyanide poisoning". Clinical Toxicology. 49 (5): 366–73. doi:10.3109/15563650.2011.584879. PMC 3882312. PMID 21740135.
- Belani, KG; Singh, H; Beebe, DS; George, P; Patterson, SE; Nagasawa, HT; Vince, R (2012). "Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium". Anesthesia and Analgesia. 114 (5): 956–61. doi:10.1213/ANE.0b013e31824c4eb5. PMC 3334426. PMID 22392971.
- ^ "New Antidote for Cyanide Found". Yahoo News. February 1, 2013.