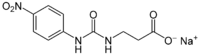
| |
| Names | |
|---|---|
| IUPAC name Sodium N--β-alaninate | |
| Systematic IUPAC name Sodium 3-{amino}propanoate | |
| Other names N-(((4-Nitrophenyl)amino)carbonyl)-β-alanine monosodium salt | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C10H10N3NaO5 |
| Molar mass | 275.196 g·mol |
| Melting point | 240 °C (464 °F; 513 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Suosan is calorie-free artificial sweetener derived from β-alanine, discovered in 1948 by Petersen et Muller.
Suosan is a sodium salt of p-Nitrophenylcarbamidopropionic acid and is 700 times sweeter than sucrose (table sugar) with a bitter aftertaste. It was never commercialized due to its low solubility in water, particularly under acidic pH (which limited its use, particularly in soft drinks) and concerns that it might form the toxic compound 4-nitroaniline.
See also
References
- Petersen S; Muller E (1948). "Über eine neue Gruppe von Süsstoffen (On a new group of sweet substances)". Chemische Berichte. 81: 31–38. doi:10.1002/cber.19480810105.
- Santhosh, C.; Mishra, P. C. (1994). "Electrostatic potential and electric field mapping of some sweeteners of the suosan series: A search for the structure-activity relationship". International Journal of Quantum Chemistry. 51 (5): 335. doi:10.1002/qua.560510510.
- AD Kinghorn & CM Compadre (2001). "Less common high-potency sweeteners". In Marcel Dekker (ed.). Alternative Sweeteners (Third ed.). New York. pp. 208–234. ISBN 0-8247-0437-1.
{{cite book}}: CS1 maint: location missing publisher (link) - Muller, George W; Culberson, J. Chris; Roy, Glenn; Ziegler, Jeanette; Walters, D. Eric; Kellogg, Michael S.; Schiffman, Susan S.; Warwick, Zoe S (May 1992). "Carboxylic acid replacement structure-activity relationships in suosan type sweeteners. A sweet taste antagonist. 1". J. Med. Chem. 35 (10): 1747–1751. doi:10.1021/jm00088a008. PMID 1588556.
- ^ Nofre, Claude; Tinti, Jean M; Chatzopoulos, Farroudja O (Mar 5, 1991). "Pyridinyl compounds of N-carbamoyl-N-thiocarbamoyl- or N-amidino-glycine or beta-alanine useful as sweetening agents. US Patent 4997667 A". Retrieved 14 September 2014.
External links
 Media related to Suosan at Wikimedia Commons
Media related to Suosan at Wikimedia Commons
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |