The synthesis of precious metals involves the use of either nuclear reactors or particle accelerators to produce these elements.
Precious metals occurring as fission products
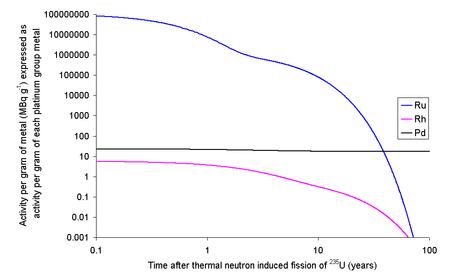
Ruthenium and rhodium are precious metals produced as a small percentage of the fission products from the nuclear fission of uranium. The longest half-lives of the radioisotopes of these elements generated by nuclear fission are 373.59 days for ruthenium and 45 days for rhodium. This makes the extraction of the non-radioactive isotope from spent nuclear fuel possible after a few years of storage, although the extract must be checked for radioactivity from trace quantities of other elements before use.
Ruthenium
See also: Airborne radioactivity increase in Europe in autumn 2017Each kilogram of the fission products of U will contain 63.44 grams of ruthenium isotopes with halflives longer than a day. Since a typical used nuclear fuel contains about 3% fission products, one ton of used fuel will contain about 1.9 kg of ruthenium. The Ru and Ru will render the fission ruthenium very radioactive. If the fission occurs in an instant then the ruthenium thus formed will have an activity due to Ru of 109 TBq g and Ru of 1.52 TBq g. Ru has a half-life of about 39 days meaning that within 390 days it will have effectively decayed to the only stable isotope of rhodium, Rh, well before any reprocessing is likely to occur. Ru has a half-life of about 373 days, meaning that if the fuel is left to cool for 5 years before reprocessing only about 3% of the original quantity will remain; the rest will have decayed. For comparison, the activity in natural potassium (due to naturally occurring
K) is about 30 Bq per gram.
Rhodium
It is possible to extract rhodium from used nuclear fuel: 1 kg of fission products of U contains 13.3 grams of Rh. At 3% fission products by weight, one ton of used fuel will contain about 400 grams of rhodium. The longest lived radioisotope of rhodium is Rh with a half-life of 2.9 years, while the ground state (Rh) has a half-life of 207 days.
Each kilogram of fission rhodium will contain 6.62 ng of Rh and 3.68 ng of Rh. As Rh decays by beta decay to either Ru (80%) (some positron emission will occur) or Pd (20%) (some gamma ray photons with about 500 keV are generated) and the excited state decays by beta decay (electron capture) to Ru (some gamma ray photons with about 1 MeV are generated). If the fission occurs in an instant then 13.3 grams of rhodium will contain 67.1 MBq (1.81 mCi) of Rh and 10.8 MBq (291 μCi) of Rh. As it is normal to allow used nuclear fuel to stand for about five years before reprocessing, much of this activity will decay away leaving 4.7 MBq of Rh and 5.0 MBq of Rh. If the rhodium metal was then left for 20 years after fission, the 13.3 grams of rhodium metal would contain 1.3 kBq of Rh and 500 kBq of Rh. Rhodium has the highest price of these precious metals ($440,000/kg in 2022), but the cost of the separation of the rhodium from the other metals needs to be considered, although recent high prices may create opportunity for consideration.
Precious metals produced via irradiation
Gold
Chrysopoeia, the artificial production of gold, is the traditional goal of alchemy. Such transmutation is possible in particle accelerators or nuclear reactors, although the production cost is estimated to be a trillion times the market price of gold. Since there is only one stable gold isotope, Au, nuclear reactions must create this isotope in order to produce usable gold.
Gold was synthesized from mercury by neutron bombardment in 1941, but the isotopes of gold produced were all radioactive. In 1924, a German scientist, Adolf Miethe, reported achieving the same feat, but after various replication attempts around the world, it was deemed an experimental error.
In 1980, Glenn Seaborg transmuted several thousand atoms of bismuth into gold at the Lawrence Berkeley Laboratory. His experimental technique was able to remove protons and neutrons from the bismuth atoms. Seaborg's technique was far too expensive to enable the routine manufacture of gold but his work is the closest yet to emulating an aspect of the mythical Philosopher's stone.
See also
References
- ^ Bush, R. P. (1991). "Recovery of Platinum Group Metals from High Level Radioactive Waste" (PDF). Platinum Metals Review. 35 (4): 202–208. doi:10.1595/003214091X354202208. Archived from the original (PDF) on 2007-09-27.
- Bin Samat, S.; Green, S.; Beddoe, A. H. (1997). "The K activity of one gram of potassium". Physics in Medicine and Biology. 42 (2): 407–413. Bibcode:1997PMB....42..407S. doi:10.1088/0031-9155/42/2/012. PMID 9044422. S2CID 250778838.
- "Rhodium Spot Prices Per Ounce Today, Live Bullion Price Chart USD". Money Metals Exchange. Retrieved 2022-06-23.
- Matson, John (January 31, 2014). "Fact or Fiction?: Lead Can Be Turned Into Gold". Scientific American. Retrieved June 21, 2024.
- R. Sherr; K. T. Bainbridge & H. H. Anderson (1941). "Transmutation of Mercury by Fast Neutrons". Physical Review. 60 (7): 473–479. Bibcode:1941PhRv...60..473S. doi:10.1103/PhysRev.60.473.
- A.Miethe, "Der Zerfall des Quecksilberatoms", Naturwissenschaften, 12(1924): 597-598
- "GOLD FROM MERCURY IMPOSSIBLE; New York University Tests Show That Transmutation Method Does Not Work. MIETHE PROCESS USED His Discovery of Gold Traces Laid to Use of Spanish Mercury, Which Contains Gold". The New York Times. October 20, 1925. Retrieved September 9, 2023.
- Nelson, Robert A. "Adept Alchemy. Part II. Chapter 7. Transmutations of Mercury". Retrieved September 9, 2023.
- Aleklett, K.; Morrissey, D.; Loveland, W.; McGaughey, P.; Seaborg, G. (1981). "Energy dependence of Bi fragmentation in relativistic nuclear collisions". Physical Review C. 23 (3): 1044. Bibcode:1981PhRvC..23.1044A. doi:10.1103/PhysRevC.23.1044.
- Matthews, Robert (December 2, 2001). "The Philosopher's Stone". The Daily Telegraph. Retrieved September 22, 2020.
External links
- Spallation Neutron Source
- Mercury 197
- Mercury 197 decays to Gold 197
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part I PART I: General Considerations and Basic Chemistry" (PDF). Platinum Metals Review. 47 (2): 74–87. doi:10.1595/003214003X4727487. Archived from the original (PDF) on 2011-06-09.
- Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part II: Separation Process" (PDF). Platinum Metals Review. 47 (2): 123–131. doi:10.1595/003214003X473123131. Archived from the original (PDF) on 2011-06-09.
- Kolarik, Zdenek; Renard, Edouard V. (2005). "Potential Applications of Fission Platinoids in Industry". Platinum Metals Review. 49 (2): 79. doi:10.1595/147106705X35263.