| |
| Names | |
|---|---|
| Preferred IUPAC name 4-benzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 3564473 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H25NO3 |
| Molar mass | 351.446 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tamibarotene (brand name: Amnolake), also called retinobenzoic acid, is orally active, synthetic retinoid, developed to overcome all-trans retinoic acid (ATRA) resistance, with potential antineoplastic activity against acute promyelocytic leukaemia (APL) . It is currently marketed only in Japan and early trials have demonstrated that it tends to be better tolerated than ATRA. Tamibarotene has been tested in many other cancer types, including Acute Myeloid Leukemia where it shows no benefit, and lung cancer, where it accelerated the cancer growth and increased mortality.
It has also been investigated as a possible treatment for Alzheimer's disease, multiple myeloma and Crohn's disease. Tamibarotene shows a similar side effect profile to Tretinoin, but exhibits lower clearance, resulting in improved dosing regiments. Hyperlipidemia is a common finding from Tamibarotene dosing.
Synthesis
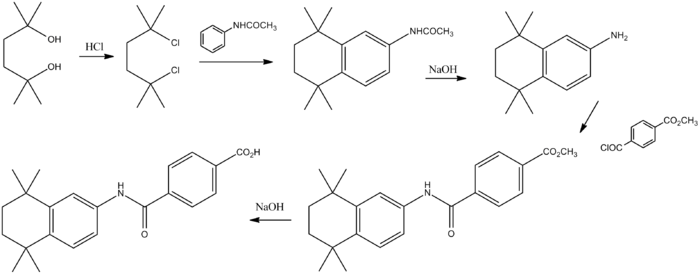
Reaction of the diol (1) with hydrogen chloride affords the corresponding dichloro derivative (2). Aluminum chloride mediated Friedel–Crafts alkylation of acetanilide with the dichloride affords the tetralin (3). Basic hydrolysis leads to the primary amine (4). Acylation of the primary amino group with the half acid chloride half ester from terephthalic acid (5) leads to the amide (6). Basic hydrolysis of the ester grouping then affords (7).
References
- "Tamibarotene: AM 80, retinobenzoic acid, Tamibaro". Drugs in R&D. 5 (6): 359–62. 2004. doi:10.2165/00126839-200405060-00010. PMID 15563242. S2CID 34546131.
- ^ Miwako, I; Kagechika, H (August 2007). "Tamibarotene". Drugs of Today. 43 (8): 563–568. doi:10.1358/dot.2007.43.8.1072615. PMID 17925887.
- "Syros Provides Update on SELECT-AML-1 Phase 2 Clinical Trial :: Syros Pharmaceuticals, Inc. (SYRS)". Syros Pharmaceuticals, Inc. 2024-08-12. Retrieved 2024-08-15.
- Arrieta, Oscar; Levitt, Daniel J.; Pendergrass, Kelly B.; Gladkov, Oleg; Bondarenko, Igor; Jain, Minish Mahendra; Wieland, Scott (2015-05-20). "Efficacy and safety of adding the retinoid tamibarotene or placebo to paclitaxel/carboplatin for advanced non-small cell lung cancer". Journal of Clinical Oncology. 33 (15_suppl): e19034. doi:10.1200/jco.2015.33.15_suppl.e19034. ISSN 0732-183X.
- Fukasawa, H; Nakagomi, M; Yamagata, N; Katsuki, H; Kawahara, K; Kitaoka, K; Miki, T; Shudo, K (2012). "Tamibarotene: a candidate retinoid drug for Alzheimer's disease" (PDF). Biological & Pharmaceutical Bulletin. 35 (8): 1206–1212. doi:10.1248/bpb.b12-00314. PMID 22863914.
- Arrieta, Oscar; Levitt, Daniel J.; Pendergrass, Kelly B.; Gladkov, Oleg; Bondarenko, Igor; Jain, Minish Mahendra; Wieland, Scott (2015-05-20). "Efficacy and safety of adding the retinoid tamibarotene or placebo to paclitaxel/carboplatin for advanced non-small cell lung cancer". Journal of Clinical Oncology. 33 (15_suppl): e19034. doi:10.1200/jco.2015.33.15_suppl.e19034. ISSN 0732-183X.
- Y. Hamada, I. Yamada, M. Uenaka, T. Sakata, U.S. patent 5,214,202 (1993).
| Carotenoids | |
|---|---|
| Carotenes (C40) | |
| Xanthophylls (C40) | |
| Apocarotenoids (C<40) | |
| Vitamin A retinoids (C20) | |
| Retinoid drugs | |
| Retinoid receptor modulators | |
|---|---|
| RARTooltip Retinoic acid receptor |
|
| RXRTooltip Retinoid X receptor | |
| |
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |