| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.025.835 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
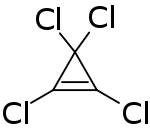
| Chemical formula | C3Cl4 |
| Molar mass | 177.83 g·mol |
| Appearance | Colorless liquid |
| Density | 1.45 g/mL |
| Boiling point | 125 - 130 C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tetrachlorocyclopropene is a chemical compound with the formula C3Cl4. A colorless liquid, the compound is a reagent used to prepare acetylene derivatives and in organic synthesis. It is prepared by addition of dichlorocarbene to trichloroethylene. It can react with water and alcohols rapidly.
The compound is used to prepare arylpropiolic acids:
- C3Cl4 + ArH + 2 H2O → ArC2CO2H + 4 HCl
Under some circumstances, diarylation occurs, giving diarylcyclopropenones, which decarbonylate to give diarylacetylenes. These reactions are thought to proceed via the intermediacy of trichlorocyclopropenium electrophile (C3Cl3).
References
- Oliver Reiser; Armin de Meijere (2001). "Tetrachlorocyclopropene". EEROS. doi:10.1002/047084289X.rt028. ISBN 0-471-93623-5.
- Glück, C; Poingée, V; Schwager, H (1987). "Improved Synthesis of 7,7-Difluorocyclopropabenzene". Synthesis. 1987 (3): 260–262. doi:10.1055/s-1987-27908. S2CID 96607067.
- Pentachlorocyclopropane Stephen W. Tobey and Robert West. The University of Wisconsin (1965)